Abstract
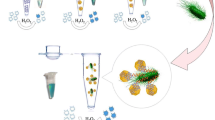
Recently, frequent outbreaks of foodborne diseases have attracted increasing attention, and how to rapidly detect foodborne pathogens has also become an urgent issue. Herein, we reported the colorimetric sensor based on chitosan-coated magnetic nanoparticles (CS@MNPs) for rapidly broad-spectrum detection of the total bacterial count, whose color change can be visible to the naked eye without any other sophisticated instruments. CS@MNPs exhibited good capture ability (greater than 80%) for six model strains, including Escherichia coli O157:H7, Salmonella enterica serovar typhimurium, Staphylococcus aureus, Listeria monocytogenes, Bacillus cereus and Cronobacter sakazakii, due to their interaction with chitosan. Under optimized conditions, the colorimetric sensor showed a linear relationship of mixed bacteria within the concentration ranging from 2.30 × 101 to 2.30 × 107 CFU·mL−1 in pure culture, and 8.50 × 101 to 8.50 × 106 CFU·mL−1 in spiked raw milk within 15 min. Besides, compared with the traditional one, colorimetric method is a more rapid and efficient method that can be applied to the dairy industry to detect the total bacterial count. In our perception, the colorimetric sensor developed in this work provides a possibility for on-set detection of the total bacterial count in other research fields.







Similar content being viewed by others
References
Organization WH (2014) ZH mediacentre news 2014: WHO’s first global report on antibiotic resistance reveals serious, worldwide threat to public health. World Health Organization, Geneva
Badalyan G, Diaz C, Bucking M, Lipski A (2018) Novel sensor platform for rapid detection and quantification of coliforms on food contact surfaces. J Microbiol Methods 153:74–83. https://doi.org/10.1016/j.mimet.2018.09.009
Meyer-Broseta S, Diot A, Bastian S, Rivière J, Cerf O (2003) Estimation of low bacterial concentration: Listeria monocytogenes in raw milk. Int J Food Microbiol 80(1):1–15
Yaghubi F, Zeinoddini M, Saeedinia AR, Azizi A, Nemati AS (2020) Design of Localized Surface Plasmon Resonance (LSPR) Biosensor for Immunodiagnostic of E. coli O157:H7 Using Gold Nanoparticles Conjugated to the Chicken Antibody. Plasmonics:1–7
Chen S, Yang X, Fu S, Qin X, Jiang Y (2020) A novel AuNPs colorimetric sensor for sensitively detecting viable Salmonella typhimurium based on dual aptamers. Food Control 115:107281
Chang YC, Yang CY, Sun RL, Cheng YF, Kao WC, Yang PC (2013) Rapid single cell detection of Staphylococcus aureus by aptamer-conjugated gold nanoparticles. Sci Rep 3:1863. https://doi.org/10.1038/srep01863
Li F, Aguilar ZP, Xiong Y, Xu H (2018) Polyamidoamine (PAMAM) dendrimer-mediated biotin amplified immunomagnetic separation method coupled with flow cytometry for viable Listeria monocytogenes detection. Sensors Actuators B Chem
Li Q, Xie G, Wang Y, Aguilar ZP, Xu H (2020) Vancomycin-modified poly-l-lysine magnetic separation combined with multiplex polymerase chain reaction assay for efficient detection of Bacillus cereus in milk. J Dairy Sci
Hol O, Parra-Flores J, Lepuschitz S, Alarcón-Lavín P, Forsythe S (2020) Molecular characterization of Cronobacter sakazakii strains isolated from powdered milk. Foods 10(20):17
Hovhannisyan VA, Bazukyan IL, Gasparyan VK (2017) Application of silver nanoparticles and CdSe quantum dots sensitized with of C-like lectin for detection of St. aureus. Comparison of various approaches. Talanta 175:366
Choi J, Chua B, Son A (2021) Ozonation enhancement of low cost double-stranded DNA binding dye based fluorescence measurement of total bacterial load in water. RSC Adv 11(7):3931–3941
ISO 4833:2013, Microbiology of the food chain - horizontal method for the enumeration of microorganisms.
Schraft H, Watterworth LA (2005) Enumeration of heterotrophs, fecal coliforms and Escherichia coli in water: comparison of 3M Petrifilm plates with standard plating procedures. J Microbiol Methods 60(3):335–342
Mao CP, Xue CF, Wang XZ, He SB, Wu LN, Yan XM (2020) Rapid quantification of pathogenic Salmonella typhimurium and total bacteria in eggs by nano-flow cytometry. Talanta 217. ARTN 12102010.1016/j.talanta.2020.121020
Xiao XL, Zhang L, Wu H, Yu YG, Tang YQ, Liu DM, Li XF (2014) Simultaneous detection of Salmonella, Listeria monocytogenes, and Staphylococcus aureus by multiplex real-time PCR assays using high-resolution melting. Food Anal Methods 7(10):1960–1972
Pinto D, Santos MA, Chambel L (2015) Thirty years of viable but nonculturable state research: unsolved molecular mechanisms. Crit Rev Microbiol 41(1):61
Law WF, Mutalib NA, Chan KG, Lee LH (2015) Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol 5:770
Sun J, Huang J, Li Y, Lv J, Ding X (2019) A simple and rapid colorimetric bacteria detection method based on bacterial inhibition of glucose oxidase-catalyzed reaction. Talanta 197:304–309
Imran M, Ehrhardt CJ, Bertino MF, Shah MR, Yadavalli V (2020) Chitosan stabilized silver nanoparticles for the electrochemical detection of lipopolysaccharide: a facile biosensing approach for gram-negative bacteria. Micromachines 11 (4)
Hong QA, Xs A, Liang YB, Ke LC, Jw A, Jc A, Fei YA, Hx A, Hx A (2020) Multiplex real-time PCR coupled with sodium dodecyl sulphate and propidium monoazide for the simultaneous detection of viable Listeria monocytogenes, Cronobacter sakazakii, Staphylococcus aureus and Salmonella spp. in milk - ScienceDirect. Int Dairy J
Deininger RA, Lee JY (2001) Rapid determination of bacteria in drinking water using an ATP assay. Field Anal Chem Technol 5 (4)
Hendrickson OD, Nikitushkin VD, Zherdev AV, Dzantiev BB (2019) Lectin-based detection of Escherichia coli and Staphylococcus aureus by flow cytometry. Arch Microbiol
Pahlow S, Meisel S, Cialla-May D, Weber K, R?Sch P, Popp J (2015) Isolation and identification of bacteria by means of Raman spectroscopy. Adv Drug Deliv Rev:105–120
Neelja S, Manish K, Kanaujia PK, Virdi JS (2015) MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol 6 (791)
Wu X, Lai T, Jiang J, Ma Y, Li N (2019) An on-site bacterial detection strategy based on broad-spectrum antibacterial ε-polylysine functionalized magnetic nanoparticles combined with a portable fluorometer. Microchimica Acta 186 (8)
Thiramanas R, Laocharoensuk R (2016) Competitive binding of polyethyleneimine-coated gold nanoparticles to enzymes and bacteria: a key mechanism for low-level colorimetric detection of gram-positive and gram-negative bacteria. Microchim Acta 183(1):389–396
Qian L, Yun X, Ning G, Cao Y, Li T, Chen Y (2017) A facile colorimetric aptamer assay for small molecule detection in food based on a magnetic single-stranded DNA binding protein-linked composite probe. Sensors Actuators B Chem 239(FEB):979–987
Shebl RI, Farouk F, Azzazy HME-S (2017) Effect of surface charge and hydrophobicity modulation on the antibacterial and antibiofilm potential of magnetic iron nanoparticles. J Nanomater 2017:3528295. https://doi.org/10.1155/2017/3528295
He A, Kf A, Mk B (2020) Synthesis of new environmentally friendly poly(urethane-imide)s as an adsorbent including β-cyclodextrin cavities and attached to iron nanoparticles for removal of gram-positive and gram-negative bacteria from water samples - ScienceDirect. Polym Test 90
Yuvaraj H, Dian K, Dirk T, Jae-Jin S (2015) Properties of chitosan/magnetite nanoparticles composites for efficient dye adsorption and antibacterial agent. Korean J Chem Eng
Adegoke O, Zolotovskaya S, Abdolvand A, Daeid NN (2020) Biomimetic graphene oxide-cationic multi-shaped gold nanoparticle-hemin hybrid nanozyme: tuning enhanced catalytic activity for the rapid colorimetric apta-biosensing of amphetamine-type stimulants. Talanta 216
Young PJ, Young JH, Il KM, Jung PT (2015) Colorimetric detection system for salmonella typhimurium based on peroxidase-like activity of magnetic nanoparticles with DNA Aptamers. J Nanomater 2015:1–9
Gao X, Yao X, Zhong Z, Jia L (2018) Rapid and sensitive detection of Staphylococcus aureus assisted by polydopamine modified magnetic nanoparticles. Talanta Int J Pure Appl Anal Chem
Jin YJ, Deng J, Liang JL, Shan C, Tong MP (2015) Efficient bacteria capture and inactivation by cetyltrimethylammonium bromide modified magnetic nanoparticles. Colloid Surface B 136:659–665. https://doi.org/10.1016/j.colsurfb.2015.10.009
Jin Y, Fei L, Chao S, Tong M, Hou Y (2014) Efficient bacterial capture with amino acid modified magnetic nanoparticles. Water Res 50:124–134
Sun Y, Zhang D, Qi P, Zheng L, Wan Y (2017) Lectin functionalized ZnO nanoarrays as a 3D nano-biointerface for bacterial detection. Talanta Int J Pure Appl Anal Chem
Yang G, Meng X, Wang Y, Yan M, Aguilar ZP, Xu H (2019) 2-Step lectin-magnetic separation (LMS) strategy combined with AuNPs-based colorimetric system for S. aureus detection in blood. Sens Actuators B Chem 279:87–94
Cuong ND, Hoa TT, Khieu DQ, Lam TD, Hoa ND, Hieu NV (2012) Synthesis, characterization, and comparative gas-sensing properties of Fe2O3 prepared from Fe3O4 and Fe3O4-chitosan. J Alloys Compd 523:120–126
Huang R, Yang B, Zheng D, Wang B (2012) Preparation and characterization of a quaternized chitosan. J Mater Sci 47(2):845–851
Konwar A, Chowdhury D, Dan A (2019) Chitosan based in situ and ex situ magnetic iron oxide nanoparticles for rapid endotoxin removal from protein solutions. Mater Chem Front
Ke L, Ki-Baek J, You SM, Da-Hee L, Jong-Yun J, Young-Rok K (2018) Surface-engineered starch magnetic microparticles for highly effective separation of a broad range of bacteria. ACS Sustain Chem Eng 6:acssuschemeng.8b03611-
Dobbins JJ (2010) Prescott's Microbiology Eighth Edition. J Microbiol Biol Educ Jmbe 11 (1):64
Guo Z, Xing R, Song L, Zhong Z, Xia J, Lin W, Li P (2008) The influence of molecular weight of quaternized chitosan on antifungal activity. Carbohyd Polym 71(4):694–697
Le TN, Tai DT, Kim MI (2020) A convenient colorimetric bacteria detection method utilizing chitosan-coated magnetic nanoparticles. Nanomaterials 10(1):92
ISO 707:2008, Milk and milk products - guidance on sampling.
ISO 16140:2016, Microbiology of the food chain - method validation.
ISO/IEC 17043:2010, Conformity assessment - general requirements for proficiency testing.
Vijayalekshmi V, Khastgir D (2017) Eco-friendly methanesulfonic acid and sodium salt of dodecylbenzene sulfonic acid doped cross-linked chitosan based green polymer electrolyte membranes for fuel cell applications. J Membr Sci 523:45–59
Shan S, Ziqing Z, Weihua L, Yonghua X, Xi C (2014) Immunomagnetic nanobeads based on a streptavidin-biotin system for the highly efficient and specific separation of Listeria monocytogenes. Food Control
China (2010). vol GB 19301-2010. Ministry of Health of the People's Republic of China, National food safety standard Raw milk
Washington (2015). Department of Health and human Services, Grade “A” pasteurized milk ordinance
Brussel: Official Journal of European Union (2004). European Union, Regulation (EC) No 853/2004 of the Europeanparliament and of the councilof 29 April 2004-laying down specific hygiene rules for food of animal origin
Canada (2015). Canadian Government, National Dairy Code Production and Processing Requirements-Part I Seventh edition revision
Canberra: Office of Legislative (2015). Attorney-General’s Department, Guide to the requirements for raw milk cheese in standard 4. 2. 4-prinmary production and processing standard for dairy products (at approval)-proposal P1022.
Burnham KP, Anderson DR (2005) Advances in ranking and selection, multiple comparisons, and reliability : methodology and applications. Advances in ranking and selection, multiple comparisons and reliablility.
Yu MX, Wu LN, Huang TX, Wang S, Yan XM (2015) Rapid detection and enumeration of total bacteria in drinking water and tea beverages using a laboratory-built high-sensitivity flow cytometer. Anal Methods 7(7):3072–3079. https://doi.org/10.1039/c4ay02919d
Funding
This study was funded by the National Natural Science Foundation of China [No. 32172320] and the Natural Science Foundation of Guangdong Province [No. 2021A1515011068].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Luying Wang declares no conflict of interest. Hong Bai declares no conflict of interest. Ximin Liu declares no conflict of interest. Xinglong Xiao declares no conflict of interest. Yigang Yu declares no conflict of interest. Xiaofeng Li declares no conflict of interest.
Ethics approval
This work does not include any experimental research on human participants and animals.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Bai, H., Liu, X. et al. Colorimetric sensor based on peroxidase-like activity of chitosan coated on magnetic nanoparticles for rapid detection of the total bacterial count in raw milk. Eur Food Res Technol 248, 1321–1333 (2022). https://doi.org/10.1007/s00217-022-03970-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-03970-8