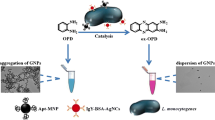
Abstract
The article describes a simple and rapid method for colorimetric detection of bacteria. It is based on competitive binding of positively charged polyethyleneimine-coated gold nanoparticles (PEI-AuNPs) to negatively charged enzymes and bacteria. The PEI-AuNPs are electrostatically attracted by both the bacterial surface and the enzyme β-galactosidase (β-Gal). Binding to the latter results in the inhibition of enzyme activity. However, in the presence of a large number of bacteria, the PEI-AuNPs preferentially bind to bacteria. Hence, the enzyme will not be inhibited and its activity can be colorimetrically determined via hydrolysis of the chromogenic substrate chlorophenol red β-D-galactopyranoside (CPRG). The detection limit of this assay is as low as 10 cfu·mL−1, and the linear range extends from 106 to 108 cfu·mL−1. The assay is applicable to both Gram-negative (such as enterotoxigenic Escherichia coli; ETEC) and Gram-positive (Staphylococcus aureus; S. aureus) bacteria. Results are obtained within 10 min using an optical reader, and within 2–3 h by bare-eye detection. The method was applied to the identification of ETEC contamination at a level of 10 cfu·mL−1 in spiked drinking water. Given its low detection limit and rapidity (sample preconcentration is not required), this method holds great promise for on-site detection of total bacterial contamination.

The method is based on competitive binding of positively charged polyethyleneimine-coated gold nanoparticles to negatively charged enzymes and bacteria. The detection limit is as low as 10 cfu·mL−1, and the linear range extends from 106 to 108 cfu·mL−1.






Similar content being viewed by others

References
Maalouf R, Fournier-Wirth C, Coste J, Chebib H, Saïkali Y, Vittori O, Errachid A, Cloarec J-P, Martelet C, Jaffrezic-Renault N (2007) Label-free detection of bacteria by electrochemical impedance spectroscopy: comparison to surface plasmon resonance. Anal Chem 79:4879–4886
Diarrhoeal. World Health Organization Homepage. http://www.who.int/vaccine_research/diseases/diarrhoeal/en/index4.html. Accessed 20 March 2013
Boyd RF (1988) General microbiology, 2nd edn. Times Mirror/Mosby College Publishing, St. Louis, pp 401–404
Sanvicens N, Pastells C, Pascual N, Marco MP (2009) Nanoparticle-based biosensors for detection of pathogenic bacteria. TrAC, Trend Anal Chem 28:1243–1252
Lui C, Cady NC, Batt CA (2009) Nucleic acid-based detection of bacterial pathogens using integrated microfluidic platform systems. Sensors 9:3713–3744
Wang Y, Deng M, Jia L (2014) N-methylimidazolium functionalized magnetic particles as adsorbents for rapid and efficient capture of bacteria. Microchim Acta 181:1275–1283
Park J, Park S, Kim Y-K (2010) Multiplex detection of pathogens using an immunochromatographic assay strip. BioChip J 4:305–312
Zhao X, Hilliard LR, Mechery SJ, Wang Y, Bagwe RP, Jin S, Tan W (2004) A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc Natl Acad Sci U S A 101:15027–15032
Hahn MA, Tabb JS, Krauss TD (2005) Detection of single bacterial pathogens with semiconductor quantum dots. Anal Chem 77:4861–4869
Scindia Y, Silbert L, Volinsky R, Kolusheva S, Jelinek R (2007) Colorimetric detection and fingerprinting of bacteria by glass-supported lipid/polydiacetylene films. Langmuir 23:4682–4687
Duan YF, Ning Y, Song Y, Deng L (2014) Fluorescent aptasensor for the determination of Samonella typhimurium based on a graphene oxide platform. Microchim Acta 181:647–653
Serra B, Morales MD, Zhang J, Reviejo AJ, Hall EH, Pingarron JM (2005) In-a-day electrochemical detection of coliforms in drinking water using a tyrosinase composite biosensor. Anal Chem 77:8115–8121
Shabani A, Zourob M, Allain B, Marquette CA, Lawrence MF, Mandeville R (2008) Bacteriophage-modified microarrays for the direct impedimetric detection of bacteria. Anal Chem 80:9475–9482
Subramanian A, Irudayaraj J, Ryan T (2006) A mixed self-assembled monolayer-based surface plasmon immunosensor for detection of E. coli O157:H7. Biosens Bioelectron 21:998–1006
Lan M, Wu J, Liu W, Zhang W, Ge J, Zhang H, Sun J, Zhao W, Wang P (2012) Copolythiophene-derived colorimetric and fluorometric sensor for visually supersensitive determination of lipopolysaccharide. J Am Chem Soc 134:6685–6694
Sun J, Ge J, Liu W, Wang X, Fan Z, Zhao W, Zhang H, Wang P, Lee S-T (2012) A facile assay for direct colorimetric visualization of lipopolysaccharides at low nanomolar level. Nano Res 5:486–493
Jokerst JC, Adkins JA, Bisha B, Mentele MM, Goodridge LD, Henry CS (2012) Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal Chem 84:2900–2907
Hossain SMZ, Ozimok C, Sicard C, Aguirre SD, Ali MM, Li Y, Brennan JD (2012) Multiplexed paper test strip for quantitative bacterial detection. Anal Bioanal Chem 403:1567–1576
Su H, Ma Q, Shang K, Liu T, Yin H, Ai S (2012) Gold nanoparticles as colorimetric sensor: a case study on E. coli O157:H7 as a model for gram-negative bacteria. Sensors Actuators B 161:298–303
Lim S, Koo OK, You YS, Lee YE, Kim M-S, Chang P-S, Kang DH, Yu J-H, Choi YJ, Gunasekaran S (2012) Enhancing nanoparticle-based visible detection by controlling the extent of aggregation. Sci Rep 2:Art. No. 456
Miranda OR, Li X, Garcia-Gonzalez L, Zhu Z-J, Yan B, Bunz UHF, Rotello VM (2011) Colorimetric bacteria sensing using a supramolecular enzyme-nanoparticle biosensor. J Am Chem Soc 133:9650–9653
Li J, Wu L-J, Guo S-S, Fu H-E, Chen G-N, Yang H-H (2013) Simple colorimetric bacterial detection and high-throughput drug screening based on a graphene-enzyme complex. Nanoscale 5:619–623
Hayden SC, Zhao G, Saha K, Phillips RL, Li X, Miranda OR, Rotello VM, El-Sayed MA, Schmidt-Krey I, Bunz UHF (2012) Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J Am Chem Soc 134:6920–6923
Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS (2012) Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in Gram-positive and Gram-negative bacteria. Proc Natl Acad Sci U S A 109:8722–8727
Kim K, Lee HB, Lee JW, Park HK, Shin KS (2008) Self-assembly of poly(ethylenimine)-capped Au nanoparticles at a toluene-water interface for efficient surface-enhanced raman scattering. Langmuir 24:7178–7183
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22
Padmanabhan SC, McGrath J, Bardosova M, Pemble ME (2012) A facile method for the synthesis of highly monodisperse silica@gold@silica core-shell-shell particles and their use in the fabrication of three-dimensional metallodielectric photonic crystals. J Mater Chem 22:11978–11987
Matthews BW (2005) The structure of E. coli β-galactosidase. CR Biol 328:549–556
Ryter A (1988) Contribution of new cryomethods to a better knowledge of bacterial anatomy. Ann Inst Pasteur Microbiol 139:33–44
Li B, Logan BE (2004) Bacterial adhesion to glass and metal-oxide surfaces. Colloids Surf B: Biointerfaces 36:81–90
Arkhangelsky E, Gitis V (2008) Effect of transmembrane pressure on rejection of viruses by ultrafiltration membranes. Sep Purif Technol 62:619–628
Berry V, Gole A, Kundu S, Murphy CJ, Saraf RF (2005) Deposition of CTAB-terminated nanorods on bacteria to form highly conducting hybrid systems. J Am Chem Soc 127:17600–17601
Acknowledgments
This research was supported by National Nanotechnology Center (NANOTEC), the National Science and Technology Development Agency (NSTDA), Thailand. Funding program number P1011227 and P1451182.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2.55 mb)
Rights and permissions
About this article
Cite this article
Thiramanas, R., Laocharoensuk, R. Competitive binding of polyethyleneimine-coated gold nanoparticles to enzymes and bacteria: a key mechanism for low-level colorimetric detection of gram-positive and gram-negative bacteria. Microchim Acta 183, 389–396 (2016). https://doi.org/10.1007/s00604-015-1657-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1657-7