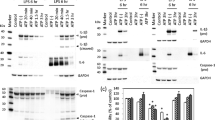
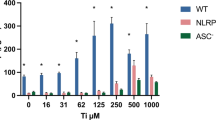
Abstract
Long fibers, such as asbestos and carbon nanotubes (CNTs), are more potent activators of inflammatory and genotoxicity than short or tangled fibers. Fibrous particles trigger interleukin (IL)-1β secretion and cause inflammatory diseases through NLRP3 inflammasomes in phagocytotic cells. However, the mechanism involved in fibrous particle-induced inflammation has not been well documented. In this study, we focused on GTPase effector Rho-kinases (ROCK1, and 2), which are known to be involved in a wide range of cellular functions such as adhesion, regulation of cytoskeleton, and phagocytosis. We examined whether ROCKs are associated with multi-walled CNT (MWCNT)- or asbestos-induced IL-1β secretion in human monocytic THP-1 cells using a selective inhibitor and small interfering RNA. THP-1 cells were differentiated to macrophages by PMA and were exposed to MWCNTs, crocidolite asbestos or lipopolysaccharide (LPS) in the presence or absence of Y27632 (ROCK inhibitor) or Z-YVAD (caspase-1 inhibitor). Exposure of the cells to MWCNTs or asbestos provoked IL-1β secretion, but this secretion was suppressed by both Y27632 and Z-YVAD, whereas LPS-induced IL-1β secretion was inhibited only by Z-YVAD and not by Y27632. siRNA designed for knockdown of both ROCK1 and ROCK2 suppressed MWCNT- and asbestos-induced IL-1β secretion, but did not change LPS-induced IL-1β secretion. Moreover, Y27632 suppressed pro-IL-1β protein levels and the release of activated-cathepsin B and activated-caspase-1 induced by MWCNTs or asbestos. In contrast, LPS-induced pro-IL-1β protein was not suppressed by Y27632. These results suggest that ROCKs are involved in fibrous particle-induced inflammasome responses in THP-1 cells.









Similar content being viewed by others
References
Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3:339–345
Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T (2011) Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 89(716–724):724A–724C
Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320:674–677
Freche B, Reig N, van der Goot FG (2007) The role of the inflammasome in cellular responses to toxins and bacterial effectors. Semin Immunopathol 29:249–260
Hirano S, Kanno S, Furuyama A (2008) Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol Appl Pharmacol 232:244–251
Hirano S, Fujitani Y, Furuyama A, Kanno S (2010) Uptake and cytotoxic effects of multi-walled carbon nanotubes in human bronchial epithelial cells. Toxicol Appl Pharmacol 249:8–15
Hirano S, Fujitani Y, Furuyama A, Kanno S (2012) Macrophage receptor with collagenous structure (MARCO) is a dynamic adhesive molecule that enhances uptake of carbon nanotubes by CHO-K1 cells. Toxicol Appl Pharmacol 259:96–103
Inoue K, Yanagisawa R, Koike E, Nishikawa M, Takano H (2010) Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: possible role of oxidative stress. Free Radic Biol Med 48:924–934
Ishizaki T, Maekawa M, Fujisawa K et al (1996) The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J 15:1885–1893
Kahlenberg JM, Dubyak GR (2004) Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol 286:C1100–C1108
Kanno S, Furuyama A, Hirano S (2007) A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicol Sci 97:398–406
Kanno S, Hirano S, Sagi M et al (2013) Sulfide induces apoptosis and Rho kinase-dependent cell blebbing in Jurkat cells. Arch Toxicol 87:1245–1256
Keka IS, Evans TJ et al (2014) A novel genotoxicity assay of carbon nanotubes using functional macrophage receptor with collagenous structure (MARCO)-expressing chicken B lymphocytes. Arch Toxicol 88:145–160
Kohyama N, Shinohara Y, Suzuki Y (1996) Mineral phases and some reexamined characteristics of the international union against cancer standard asbestos samples. Am J Ind Med 30:515–528
Leung T, Manser E, Tan L, Lim L (1995) A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem 270:29051–29054
Li M, Gunter ME, Fukagawa NK (2012) Differential activation of the inflammasome in THP-1 cells exposed to chrysotile asbestos and Libby “six-mix” amphiboles and subsequent activation of BEAS-2B cells. Cytokine 60:718–730
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241
McCarty DJ Jr, Faires JS (1963) A comparison of the duration of local anti-inflammatory effect of several adrenocorticosteroid esters—a bioassay technique. Curr Ther Res Clin Exp 5:284–290
Meunier E, Coste A, Olagnier D et al (2012) Double-walled carbon nanotubes trigger IL-1beta release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine 8:987–995
Misawa T, Takahama M, Kozaki T et al (2013) Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol 14:454–460
Mitchell JA, Paul-Clark MJ, Clarke GW, McMaster SK, Cartwright N (2007) Critical role of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. J Endocrinol 193:323–330
Mossman BT, Lippmann M, Hesterberg TW, Kelsey KT, Barchowsky A, Bonner JC (2011) Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J Toxicol Environ Health B Crit Rev 14:76–121
Nagai H, Toyokuni S (2010) Biopersistent fiber-induced inflammation and carcinogenesis: lessons learned from asbestos toward safety of fibrous nanomaterials. Arch Biochem Biophys 502:1–7
Niemi K, Teirila L, Lappalainen J et al (2011) Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol 186:6119–6128
Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM (2002) Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr Biol 12:1413–1418
Osuka A, Hanschen M, Stoecklein V, Lederer JA (2012) A protective role for inflammasome activation following injury. Shock 37:47–55
Pacurari M, Castranova V, Vallyathan V (2010) Single- and multi-wall carbon nanotubes versus asbestos: are the carbon nanotubes a new health risk to humans? J Toxicol Environ Health A 73:378–395
Palomaki J, Valimaki E, Sund J et al (2011) Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano 5:6861–6870
Perregaux D, Gabel CA (1994) Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem 269:15195–15203
Qu Y, Franchi L, Nunez G, Dubyak GR (2007) Nonclassical IL-1β secretion stimulated by P2×7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 179:1913–1925
Rajamaki K, Lappalainen J, Oorni K et al (2010) Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 5:e11765
Rock KL, Kataoka H, Lai JJ (2013) Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol 9:13–23
Sakamaki I, Inai K, Tsutani Y, Ueda T, Tsutani H (2008) Binding of monosodium urate crystals with idiotype protein efficiently promote dendritic cells to induce cytotoxic T cells. Cancer Sci 99:2268–2273
Sandberg WJ, Lag M, Holme JA et al (2012) Comparison of non-crystalline silica nanoparticles in IL-1beta release from macrophages. Part Fibre Toxicol 9:32
Schinwald A, Donaldson K (2012) Use of back-scatter electron signals to visualise cell/nanowires interactions in vitro and in vivo; frustrated phagocytosis of long fibres in macrophages and compartmentalisation in mesothelial cells in vivo. Part Fibre Toxicol 9:34
Schinwald A, Murphy FA, Prina-Mello A et al (2012) The threshold length for fiber-induced acute pleural inflammation: shedding light on the early events in asbestos-induced mesothelioma. Toxicol Sci 128:461–470
Shi J, Wei L (2007) Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz) 55:61–75
Shukla A, MacPherson MB, Hillegass J et al (2009) Alterations in gene expression in human mesothelial cells correlate with mineral pathogenicity. Am J Respir Cell Mol Biol 41:114–123
Shvedova AA, Kisin E, Murray AR et al (2008) Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol 295:L552–L565
Soldatos AN, Metheniti A, Mamali I, Lambropoulou M, Marmaras VJ (2003) Distinct LPS-induced signals regulate LPS uptake and morphological changes in medfly hemocytes. Insect Biochem Mol Biol 33:1075–1084
Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ (2010) Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS One 5:e8774
Tsuchiya S, Kobayashi Y, Goto Y et al (1982) Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res 42:1530–1536
Yazdi AS, Guarda G, Riteau N et al (2010) Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1alpha and IL-1beta. Proc Natl Acad Sci USA 107:19449–19454
Yoneda A, Multhaupt HA, Couchman JR (2005) The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol 170:443–453
Zalma R, Bonneau L, Guignard J, Pezerat H (1987) Formation of oxy radicals by oxygen reduction arising from the surface activity of asbestos. Can J Chem 65:2338–2341
Acknowledgments
We would like to thank Mr. T. Ikawa for the technical support. This work was supported by JSPS KAKENHI (Grant No. 24590868).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanno, S., Hirano, S., Chiba, S. et al. The role of Rho-kinases in IL-1β release through phagocytosis of fibrous particles in human monocytes. Arch Toxicol 89, 73–85 (2015). https://doi.org/10.1007/s00204-014-1238-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1238-2