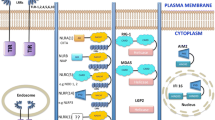
Abstract
Invading pathogens are recognized by mammalian cells through dedicated receptors found either at the cell surface or in the cytoplasm. These receptors, like the trans-membrane Toll-like Receptors (TLR) or the cytosolic Nod-like Receptors (NLR), initiate innate immunity after recognition of molecular patterns found in bacteria or viruses, such as LPS, flagellin, or double-stranded RNA. Recognition of molecules produced only by a specific pathogen, such as a viral envelop protein or a bacterial adhesin does not appear to occur. Bacterial protein toxins, however, might compose an intermediate class. Considering the diversity of toxins in terms of structure, it is unlikely that cells respond to them via specific molecular recognition. It rather appears that different classes of toxins trigger cellular changes that are sensed by the cells as danger signals, such as changes in cellular ion composition after membrane perforation by pore-forming toxins or type III secretion systems. The signaling pathways triggered through toxin-induced cell alterations will likely play a role in modulating host responses to virulent bacteria. We will here describe the few studied cases in which detection of the toxin by the host cell was addressed. The review will include not only toxins but also bacteria effectors secreted by the bacterium in to the host cell cytoplasm.


Similar content being viewed by others
References
Abrami L, Fivaz M et al (2000) Adventures of a pore-forming toxin at the target cell surface. Trends Microbiol 8(4):168–172
Abrami L, Reig N et al (2005) Anthrax toxin: the long and winding road that leads to the kill. Trends Microbiol 13(2):72–78
Akira S, Uematsu S et al (2006) Pathogen recognition and innate immunity. Cell 124(4):783–801
Alouf JE, Freer JH (eds) (2005) The comprehensive sourcebook of bacterial protein toxins. Academic Press, London
Amer A, Franchi L et al (2006) Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem 281(46):35217–35223
Arbibe L, Kim DW et al (2007) An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat Immunol 8(1):47–56
Aroian R, van der Goot FG (2007) Pore-forming toxins and cellular non-immune defenses (CNIDs). Curr Opin Microbiol 10(1):57–61
Baldari CT, Tonello F et al (2006) Anthrax toxins: a paradigm of bacterial immune suppression. Trends Immunol 27(9):434–440
Boyden ED, WF Dietrich (2006) Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet 38(2):240–244
Bradley KA, Mogridge J et al (2001) Identification of the cellular receptor for anthrax toxin. Nature 414(6860):225–229
Chaowagul W, White NJ et al (1989) Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis 159(5):890–899
Chen LM, Kaniga K et al (1996) Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol 21(5):1101–1115
Chen Y, Smith MR et al (1996) A bacterial invasin induces macrophage apoptosis by binding directly to ICE. Embo J 15(15):3853–3860
Collier RJ (1990) Diphtheria toxin: Structure and function of a cytocidal protein. ADP-ribosylating toxins and G proteins: Insights into signal transduction. J. M. a. M. Vaughan. Washington DC, American Society of Microbiology: 3–19
Cordoba-Rodriguez R, Fang H et al (2004) Anthrax lethal toxin rapidly activates caspase-1/ICE and induces extracellular release of interleukin (IL)-1beta and IL-18. J Biol Chem 279(20):20563–20566
Cossart P, Sansonetti PJ (2004) Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304(5668):242–248
Dinarello CA (1998) Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci 856:1–11
Ding Z, Atmakuri K et al (2003) The outs and ins of bacterial type IV secretion substrates. Trends Microbiol 11(11):527–535
Faustin B, Lartigue L et al (2007) Reconstituted Nalp1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell 25:713–724
Fink SL, Cookson BT (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73(4):1907–1916
Fink SL, Cookson BT (2006) Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 8(11):1812–1825
Franchi L, Amer A et al (2006) Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol 7(6):576–582
Geny B, Popoff MR (2006) Bacterial protein toxins and lipids: Pore formation or toxin entry into cells. Biol Cell 98(11):667–678
Giddings KS, Zhao J et al (2004) Human CD59 is a receptor for the cholesterol dependent cytolysin intermedilysin. Nat Struct Mol Biol 11:1173–1178
Griffitts JS, Haslam SM et al (2005) Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science 307:922–925
Goldstein JL, DeBose-Boyd RA et al (2006) Protein sensors for membrane sterols. Cell 124(1):35–46
Gurcel L, Abrami L et al (2006) Caspase-1 dependent activation of SREBPS promotes cell survival in response to bacterial pore-forming toxins. Cell 126:1135–1145
Gurcel L, Iacovache I et al (2005) Aerolysin and related Aeromonas toxins. The comprehensive sourcebook of bacterial protein toxins. JE Alouf, JH Freer, Academic, London
Haugwitz U, Bobkiewicz W et al (2006) Pore-forming Staphylococcus aureusalpha-toxin triggers epidermal growth factor receptor-dependent proliferation. Cell Microbiol 8(10):1591–1600
Hernandez LD, Hueffer K et al (2004) Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science 304(5678):1805–1807
Hersh D, Monack DM et al (1999) The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA 96(5):2396–2401
Heuck AP, Tweten RK et al (2001) Beta-barrel pore-forming toxins: intriguing dimorphic proteins. Biochemistry 40(31):9065–9073
Hilbi H, Chen Y et al (1997) The interleukin 1beta-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun 65(12):5165–5170
Hilbi H, Moss JE et al (1998) Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem 273(49):32895–32900
Huffman DL, Abrami L et al (2004) Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc Natl Acad Sci USA 101(30):10995–11000
Husmann M, Dersch K et al (2006) Differential role of p38 mitogen activated protein kinase for cellular recovery from attack by pore-forming S. aureus alpha-toxin or streptolysin O. Biochem Biophys Res Commun 344(4):1128–1134
Kanneganti TD, Lamkanfi M et al (2007) Pannexin-1-mediated recognition of bacterial molecules activates the Cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26(4):433–443
Lemaitre B, Nicolas E et al (1996) The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86(6):973–983
Lencer WI, Tsai B (2003) The intracellular voyage of cholera toxin: going retro. Trends Biochem Sci 28(12):639–645
Madden JC, Ruiz N et al (2001) Cytolysin-mediated translocation (CMT): A functional equivalent of type III secretion in gram-positive bacteria. Cell 104(1):143–152
Mariathasan S (2007) ASC, Ipaf and Cryopyrin/Nalp3: Bona fide intracellular adapters of the caspase-1 inflammasome. Microbes Infect 9(5):664–671
Mariathasan S, Monack DM (2007) Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 7(1):31–40
Mariathasan S, Newton K et al (2004) Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430(6996):213–218
Mariathasan S, Weiss DS et al (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440(7081):228–232
Martinon F, Burns K et al (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10(2):417–426
Martinon F, Tschopp J (2005) NLRs join TLRs as innate sensors of pathogens. Trends Immunol 26(8):447–454
Miao EA, Alpuche-Aranda CM et al (2006) Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 7(6):569–575
Miggin SM, O’Neill LA (2006) New insights into the regulation of TLR signaling. J Leukoc Biol 80(2):220–226
Miggin SM, Palsson-McDermott E et al (2007) NF-kappaB activation by the Toll-IL-1 receptor domain protein MyD88 adapter-like is regulated by caspase-1. Proc Natl Acad Sci USA 104(9):3372–3377
Mota LJ, Sorg I et al (2005) Type III secretion: The bacteria-eukaryotic cell express. FEMS Microbiol Lett 252(1):1–10
Muehlbauer SM, Evering TH et al (2007) Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle 6(6):758–766
Mukherjee S, Keitany G et al (2006) Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312(5777):1211–1214
Nicolaou KC, Frederick MO (2007) On the Structure of Maitotoxin. Angew Chem Int Ed Engl
Ozoren N, Masumoto J et al (2006) Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol 176(7):4337–4342
Parker MW, Feil SC (2005) Pore-forming protein toxins: From structure to function. Prog Biophys Mol Biol 88(1):91–142
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. Embo J 25(21):5071–5082
Pelegrin P, Surprenant A (2007) Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem 282(4):2386–2394
Perregaux D, Gabel CA (1994) Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem 269(21):15195–15203
Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ (in press)
Ratner AJ, Hippe KR et al (2006) Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J Biol Chem
Reig N, van der Goot FG (2006) About lipids and toxins. FEBS Lett 580(23):5572–5579
Ren T, Zamboni DS et al (2006) Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog 2(3):e18
Rosch J, Caparon M (2004) A microdomain for protein secretion in Gram-positive bacteria. Science 304(5676):1513–1515
Sandvig K, van Deurs B (2002) Transport of protein toxins into cells: Pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett 529(1):49–53
Sansonetti PJ, Phalipon A et al (2000) Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12(5):581–590
Sarkar A, Duncan M et al (2006) ASC directs NF-kappaB activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J Immunol 176(8):4979–4986
Scobie HM, Rainey GJ et al (2003) Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci USA 100(9):5170–5174
Scobie HM, Young JA (2005) Interactions between anthrax toxin receptors and protective antigen. Curr Opin Microbiol 8(1):106–112
Shin H, Cornelis GR (2007) Type III secretion translocation pores of Yersinia enterocolitica trigger maturation and release of Pro-inflammatory IL-1beta. Cell Micro (in press)
Sun GW, Lu J et al (2005) Caspase-1 dependent macrophage death induced by Burkholderia pseudomallei. Cell Microbiol 7(10):1447–1458
Sutterwala FS, Ogura Y, Flavell RA (2007) The inflammasome in pathogen recognition and inflammation. J Leukoc Biol (in press)
Troisfontaines P, Cornelis GR (2005) Type III secretion: more systems than you think. Physiology (Bethesda) 20:326–339
Tschopp J, Martinon F et al (2003) NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol 4(2):95–104
Turton K, Chaddock JA et al (2002) Botulinum and tetanus neurotoxins: Structure, function and therapeutic utility. Trends Biochem Sci 27(11):552–558
Tweten RK, Parker MW et al (2001) The cholesterol-dependent cytolysins. Curr Top Microbiol Immunol 257:15–33
Vabulas RM, Wagner H, Schild H (2002) Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol 270:169–184
van der Velden AW, Velasquez M et al (2003) Salmonella rapidly kill dendritic cells via a caspase-1-dependent mechanism. J Immunol 171(12):6742–6749
Veiga E, Cossart P (2005) Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol 7(9):894–900
Walev I, Reske K et al (1995) Potassium-inhibited processing of IL-1 beta in human monocytes. Embo J 14(8):1607–1614
Wisnoskey BJ, Estacion M et al (2004) Maitotoxin-induced cell death cascade in bovine aortic endothelial cells: divalent cation specificity and selectivity. Am J Physiol Cell Physiol 287(2):C345–C356
Yamamoto M, Yaginuma K et al (2004) ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells 9(11):1055–1067
Zamboni DS, Kobayashi KS et al (2006) The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol 7(3):318–325
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freche, B., Reig, N. & van der Goot, F.G. The role of the inflammasome in cellular responses to toxins and bacterial effectors. Semin Immunopathol 29, 249–260 (2007). https://doi.org/10.1007/s00281-007-0085-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-007-0085-0