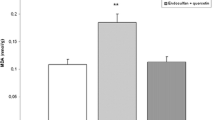
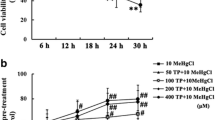
Abstract
The hypothesis that methylmercury (MeHg) potently induces formation of reactive oxygen species (ROS) in the brain is supported by observations on the neuroprotective effects of various classes of antioxidants. Flavonoids have been reported to possess divalent metal chelating properties, antioxidant activities and to readily permeate the blood–brain barrier. They can also provide neuroprotection in a wide array of cellular and animal models of neurological diseases. Paradoxically, in vivo administration of quercetin displays unexpected synergistic neurotoxic effect with MeHg. Considering this controversy and the limited data on the interaction of MeHg with other flavonoids, the potential protective effect of quercetin and two of its glycoside analogs (i.e., rutin and quercitrin) against MeHg toxicity were evaluated in rat cortical brain slices. MeHg (100 μM) caused lipid peroxidation and ROS generation. Quercitrin (10 μg/mL) and quercetin (10 μg/mL) protected mitochondria from MeHg (5 μM)-induced changes. In contrast, rutin did not afford a significant protective effect against MeHg (100 μM)-induced lipid peroxidation and ROS production in cortical brain slices. MeHg-generated ROS in cortical slices was dependent upon an increase in intracellular Ca2+ levels, because the over-production of MeHg-induced H2O2 in mitochondria occurred with a concomitant increase in Ca2+ transient. Here, we have extended the characterization of mechanisms associated with the neuroprotective effects of quercetin against MeHg-induced toxicity in isolated mitochondria, by performing an array of parallel studies in brain slices. We provide novel data establishing that (1) Ca2+ plays a central role in MeHg toxicity and (2) in brain slices MeHg induces mitochondrial oxidative stress both via direct interaction with mitochondria (as previously reported in in vitro studies) as well as via mitochondria-independent (or indirect) mechanisms.





Similar content being viewed by others
References
Allen JW, Mutkus LA, Aschner M (2001) Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res 902:92–100
Allen JW, Shanker G, Tan KH, Aschner M (2002) The consequences of methylmercury exposure on interactive functions between astrocytes and neurons. Neurotoxicology 23:755–759
Araragi S, Kondoh M, Kawas M, Saito S, Higashimoto M, Sato M (2003) Mercuric chloride induces apoptosis via a mitochondrial-dependent pathway in human leukemia cells. Toxicology 184:1–9
Aschner M, Yao CP, Allen JW, Tan KH (2000) Methylmercury alters glutamate transport in astrocyte. Neurochem Int 37:199–206
Aschner M, Syversen T, Souza DO, Rocha JBT, Farina M (2007) Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res 40:285–291
Brustovetsky N, Dubinsky JM (2000) Dual responses of CNS mitochondria to elevated calcium. J Neurosci 20:103–113
Cai Q, Rahn RO, Zhang R (1997) Dietary flavonoids, quercetin, luteolin and genistein reduce oxidative DNA damage and lipid peroxidation and quench free radicals. Cancer Lett 119:99–107
Choi DW (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury-current exposures and clinical manifestations. New Engl J Med 349:1731–1737
Cotelle N (2001) Role of flavonoids in oxidative stress. Curr Top Med Chem 1:569–590
Dajas F, Rivera-Megre F, Blasina F, Arredondo F, Abin-Carriquiry JA, Costa G, Echeverry C, Lafon L, Heizen H, Ferreira M, Morquio A (2003) Neuroprotection by flavonoids. Braz J Med Biol Res 36:1613–1620
Denny MF, Hare MF, Atchison WD (1993) Methylmercury alters intrasynaptosomal concentrations of endogenous polyvalent cations. Toxicol Appl Pharmacol 122:222–232
Dreiem A, Seegal RF (2007) Methylmercury-induced changes in mitochondrial function in striatal synaptosomes are calcium-dependent and ROS-independent. Neurotoxicology 28:720–726
Dubinsky JM, Levi Y (1998) Calcium-induced activation of the mitochondrial permeability transition in hippocampal neurons. J Neurosci Res 53:728–741
Farina M, Franco JL, Ribas CM, Meotti FC, Missau FC, Pizzolatti MG, Dafre AL, Santos AR (2005) Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J Pharm Pharmacol 57:1503–1508
Fiskum G, Rosenthal RE, Vereczky V (2004) Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr 36:347–352
Fonfria E, Vilaro MT, Babot Z, Rodriguez-Farre E, Sunol C (2005) Mercury compounds disrupt neuronal glutamate transport in cultured mouse cerebellar granule cells. J Neurosci Res 79:545–553
Franco JL, Braga HC, Stringari J, Missau FC, Posser T, Mendes BG, Leal RB, Santos AR, Dafre AL, Pizzolatti MG, Farina M (2007) Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem Res Toxicol 20:1919–1926
Grotto D, de Castro MM, Barcelos GRM, Garcia SC, Barbosa F (2009) Low level and sub-chronic exposure to methylmercury induces hypertension in rats: nitric oxide depletion and oxidative damage as possible mechanisms. Arch Toxicol 83:653–662
Gugliucci A, Stahl AJ (1995) Low density lipoprotein oxidation is inhibited by extracts of Ilex paraguariensis. Biochem Mol Biol Int 35:47–56
Gupta R, Singh M, Sharma A (2003) Neuroprotective effect of antioxidants on ischaemia and reperfusion-induced cerebral injury. Pharmacol Res 48:209–215
Hansson MJ, Månsson R, Morota S, Uchino H, Kallur T, Sumi T, Ishii N, Shimazu M, Keep MF, Jegorov A, Elmér E (2008) Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic Biol Med 45:284–294
Hollman PC, Katan MB (1999) Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol 37:937–942
InSug O, Datar S, Koch CJ, Shapiro IM, Shenker BJ (1997) Mercuric compounds inhibit human monocyte function by inducing apoptosis: evidence for formation of reactive oxygen species, development of mitochondrial membrane permeability transition and loss of reductive reserve. Toxicology 124:211–224
Kaariaien TM, Piltonen M, Ossola B (2008) Lack of robust protective effect of quercetin in two types of 6-hydroxydopamine-induced parkinsonian models in rats and dopaminergic cell cultures. Brain Res 1203:149–159
Komulainen H, Bondy SC (1987) Increased free intrasynaptosomal Ca2+ by neurotoxic organometals: distinctive mechanisms. Toxicol Appl Pharmacol 88:77–86
Lifshitz J, Sullivan PG, Hovda DA (2004) Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 4:705–713
Limke TL, Heidemann SR, Atchison WD (2004) Disruption of intraneuronal divalent cation regulation by methylmercury: are specific targets involved in altered neuronal development and cytotoxicity in methylmercury poisoning? Neurotoxicology 25:741–760
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mande S, Amit T, Reznichenk L, Weinre O, Youdim MB (2006) Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol Nutr Food Res 50:229–234
Martins RP, Braga HC, Silva AP, Dalmarco JB, de Bem AF, dos Santos AR, Dafre AL, Pizzolatti MG, Latini A, Aschner M, Farina M (2009) Synergistic neurotoxicity induced by methylmercury and quercetin in mice. Food Chem Toxicol 47:645–649
Marty MS, Atchison WD (1997) Pathways mediating Ca2+ entry in rat cerebellar granule cells following in vitro exposure to methyl mercury. Toxicol Appl Pharmacol 147:319–330
Meotti FC, Fachinetto R, Maffi LC, Missau FC, Pizzolatti MG, Rocha JB, Santos AR (2007) Antinociceptive action of myricitrin: involvement of the K+ and Ca2+ channels. Eur J Pharmacol 567:198–205
Morel AF, Dias GO, Porto C, Simionatto C, Stuker CZ, Dalcol II (2006) Antimicrobial activity of extractives of Solidago microglossa. Fitoterapia 77:453–455
Mori N, Yasutake A, Hirayama K (2007) Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Arch Toxicol 81:769–776
Pereira RP, Fachinetto R, de Souza Prestes A, Puntel RL, Santos da Silva GN, Heinzmann BM, Boschetti TK, Athayde ML, Bürger ME, Morel AF, Morsch VM, Rocha JB (2009) Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus. Neurochem Res 34:973–983
Reichl FX, Esters M, Simon S, Seiss M, Kehe K, Kleinsasser N, Folwaczny M, Glas J, Hickel R (2006a) Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch Toxicol 80:370–377
Reichl FX, Simon S, Esters M, Seiss M, Kehe K, Kleinsasser N, Hickel R (2006b) Cytotoxicity of dental composite (co)monomers and the amalgam component Hg(2+) in human gingival fibroblasts. Arch Toxicol 80:465–472
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Roos DH, Puntel RL, Santos MM, Souza DO, Farina M, Nogueira CW, Aschner M, Burger ME, Barbosa NB, Rocha JB (2009) Guanosine and synthetic organoselenium compounds modulate methylmercury-induced oxidative stress in rat brain cortical slices: Involvement of oxidative stress and glutamatergic system. Toxicol In Vitro 23:302–307
Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F (1995) Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med 19:481–486
Santamaría A, Santamaría D, Díaz-Muñoz M, Espinoza-González V, Ríos C (1997) Effects of N omega-nitro-l-arginine and l-arginine on quinolinic acid-induced lipid peroxidation. Toxicol Lett 93:117–124
Schmid K, Sassen A, Staudenmaier R, Kroemer S, Reichl FX, Harréus U, Hagen R, Kleinsasser N (2007) Mercuric dichloride induces DNA damage in human salivary gland tissue cells and lymphocytes. Arch Toxicol 81:759–767
Shanker G, Aschner M (2003) Methylmercury-induced reactive oxygen species formation in neonatal cerebral astrocytic cultures is attenuated by antioxidants. Mol Brain Res 110:85–91
Shenker BJ, Guo TL, Shapiro IM (1999) Induction of apoptosis in human T-cells by methyl mercury: temporal relationship between mitochondrial dysfunction and loss of reductive reserve. Toxicol Appl Pharmacol 157:23–35
Sirois JE, Atchison WD (2000) Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells. Toxicol Appl Pharmacol 167:1–11
Spanos GA, Wrolstad RE (1992) Phenolic of apple, pear, and white grape juices and their changes with processing and storage—a rewiew. J Agric Food Chem 40:1478–1487
Sudati JH, Fachinetto R, Pereira RP, Boligon AA, Athayde ML, Soares FA, Barbosa NV, Rocha JB (2009) In vitro antioxidant activity of Valeriana officinalis against different neurotoxic agents. Neurochem Res 34:1372–1379
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Verity MA, Brown WJ, Cheung M (1975) Organic mercurial encephalopathy: in vivo and in vitro effects of methyl mercury on synaptosomal respiration. J Neurochem 25:759–766
Wagner C, Fachinetto R, Dalla Corte CL, Brito VB, Severo D, Costa Dias G, Morel AF, Nogueira CW, Rocha JB (2006) Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res 1107:192–198
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Yee S, Choi BH (1994) Methylmercury poisoning induces oxidative stress in the mouse brain. Exp Mol Pathol 60:188–196
Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ (2004) Flavonoid permeability across an in situ model of the blood–brain barrier. Free Radic Biol Med 36:592–604
Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT (2005) Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res 39:1119–1125
Acknowledgments
The support by CAPES, CNPq, and FAPERGS is gratefully acknowledged. MA was supported by grants from NIEHS ES07331 and 10563.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wagner, C., Vargas, A.P., Roos, D.H. et al. Comparative study of quercetin and its two glycoside derivatives quercitrin and rutin against methylmercury (MeHg)-induced ROS production in rat brain slices. Arch Toxicol 84, 89–97 (2010). https://doi.org/10.1007/s00204-009-0482-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-009-0482-3