Abstract
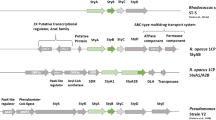
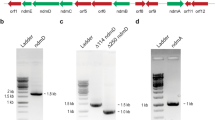
Two styrene monooxygenase types, StyA/StyB and StyA1/StyA2B, have been described each consisting of an epoxidase and a reductase. A gene fusion which led to the chimeric reductase StyA2B and the occurrence in different phyla are major differences. Identification of SMOA/SMOB-ADP1 of Acinetobacter baylyi ADP1 may enlighten the gene fusion event since phylogenetic analysis indicated both proteins to be more related to StyA2B than to StyA/StyB. SMOB-ADP1 is classified like StyB and StyA2B as HpaC-like reductase. Substrate affinity and turnover number of the homo-dimer SMOB-ADP1 were determined for NADH (24 µM, 64 s−1) and FAD (4.4 µM, 56 s−1). SMOB-ADP1 catalysis follows a random sequential mechanism, and FAD fluorescence is quenched upon binding to SMOB-ADP1 (K d = 1.8 µM), which clearly distinguishes that reductase from StyB of Pseudomonas. In summary, this study confirmes made assumptions and provides phylogenetic and biochemical data for the differentiation of styrene monooxygenase-related flavin reductases.










Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. doi:10.1016/S0022-2836(05)80360-2
Barrio JR, Tolman GL, Leonard NJ, Spencer RD, Weber G (1973) Flavin 1, N6-ethenoadenine dinucleotide: dynamic and static quenching of fluorescence. Proc Nat Acad Sci. 70:941–943. doi:10.1073/pnas.70.3.941
Beltrametti F, Marconi AM, Bestetti G, Colombo C, Galli E, Ruzzi M, Zennaro E (1997) Sequencing and functional analysis of styrene catabolism genes from Pseudomonas fluorescens ST. Appl Environ Microbiol 63:2232–2239
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Chaiyen P, Suadee C, Wilairat P (2001) A novel two-protein component flavoprotein hydroxylase. Eur J Biochem 268:5550–5561. doi:10.1046/j.1432-1033.2001.02490.x
Chakraborty S, Ortiz-Maldonado M, Entsch B, Ballou DP (2010) Studies on the mechanism of p-hydroxyphenylacetate 3-hydroxylase from Pseudomonas aeruginosa: a system composed of a small flavin reductase and a large flavin-dependent oxygenase. Biochemistry 49:372–385. doi:10.1021/bi901454u
Covès J, Nivière V, Eschenbrenner M, Fontecave M (1993) NADPH-sulfite reductase from Escherichia coli. A flavin reductase participating in the generation of the free radical of ribonucleotide reductase. J Biol Chem 268:18604–18609. doi:10.1128/JB.01050-07
Felsenstein J (1989) PHYLIP—Phylogeny Inference Package (Version 3.2). Cladistics 5:164–166
Ferreira MI, Iida T, Hasan SA, Nakamura K, Fraaije MW, Janssen DB, Kudo T (2009) Analysis of two gene clusters involved in the degradation of 4-fluorophenol by Arthrobacter sp. strain IF1. Appl Environ Microbiol 75:7767–7773. doi:10.1128/AEM.00171-09
Fromm HJ (1979) Summary of kinetic reaction mechanisms. Methods Enzymol 63:42–53
Galan B, Díaz E, Prieto MA, García JL (2000) Functional analysis of the small component of the 4-hydroxyphenylacetate 3-monooxygenase of Escherichia coli W: a prototype of a new flavin:NAD(P)H reductase subfamily. J Bacteriol 182:627–636. doi:10.1128/JB.182.3.627-636.2000
Gibello A, Suarez M, Allende JL, Martin M (1997) Molecular cloning and analysis of the genes encoding the 4-hydroxyphenylacetate hydroxylase from Klebsiella pneumoniae. Arch Microbiol 167:160–166. doi:10.1007/s002030050429
Gisi MR, Xun L (2003) Characterization of chlorophenol 4-monooxygenase (TftD) and NADH:flavin adenine dinucleotide oxidoreductase (TftC) of Burkholderia cepacia AC1100. J Bacteriol 185:2786–2792. doi:10.1128/JB.185.9.2786-2792.2003
Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. doi:10.1093/molbev/msp259
Hartmans S, van der Werf MJ, de Bont JA (1990) Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl Environ Microbiol 56:1347–1351
Hollmann F, Lin P-C, Witholt B, Schmid A (2003) Stereospecific biocatalytic epoxidation: the first example of direct regeneration of a FAD-dependent monooxygenase for catalysis. J Am Chem Soc 125:8209–8217. doi:10.1021/ja034119u
Kantz A, Gassner GT (2011) Nature of the reaction intermediates in the flavin adenine dinucleotide-dependent epoxidation mechanism of styrene monooxygenase. Biochemistry 50:523–532. doi:10.1021/bi101328r
Kantz A, Chin F, Nallamothu N, Nguyen T, Gassner GT (2005) Mechanism of flavin transfer and oxygen activation by the two-component flavoenzyme styrene monooxygenase. Arch Biochem Biophys 442:102–116. doi:10.1016/j.abb.2005.07.020
Kirchner U, Westphal AH, Müller R, van Berkel WJH (2003) Phenol hydroxylase from Bacillus thermoglucosidasius A7, a two-protein component monooxygenase with a dual role for FAD. J Biol Chem 278:47545–47553. doi:10.1074/jbc.M307397200
Lee J-K, Zhao H (2007) Identification and characterization of the flavin:NADH reductase (PrnF) involved in a novel two-component arylamine oxygenase. J Bacteriol 189:8556–8563. doi:10.1128/JB.01050-07
Lei B, Tu S-C (1998) Mechanism of reduced flavin transfer from Vibrio harveyi NADPH-FMN oxidoreductase to luciferase. Biochemistry 37:14623–14629. doi:10.1021/bi981841+
Lei B, Liu M, Huang S, Tu S-C (1994) Vibrio harveyi NADPH-flavin oxidoreductase: cloning, sequencing and overexpression of the gene and purification and characterization of the cloned enzyme. J Bacteriol 176:3552–3558
Lin H, Qiao J, Liu Y, Wu Z-L (2010) Styrene monooxygenase from Pseudomonas sp. LQ26 catalyzes the asymmetric epoxidation of both conjugated and unconjugated alkenes. J Mol Catal B Enzym 67:236–241. doi:10.1016/j.molcatb.2010.08.012
Massey V (1994) Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem 269:22459–22462
Metzgar D, Bacher JM, Pezo V, Reader J, Döring V, Schimmel P, Marlière P, De Crécy-Lagard V (2004) Acinetobacter sp. ADP1: an ideal model organism for genetic analysis and genome engineering. Nucleic Acids Res 32:5780–5790. doi:10.1093/nar/gkh881
Montersino S, Tischler D, Gassner GT, van Berkel WJH (2011) Catalytic and structural features of flavoprotein hydroxylases and epoxidases. Adv Synth Catal 353:2301–2319. doi:10.1002/adsc.201100384
Morrison E, Kantz A, Gassner GT, Sazinsky MH (2013) Structure and mechanism of styrene monooxygenase reductase: new insight into the FAD-transfer reaction. Biochemistry 52:6063–6075. doi:10.1021/bi400763h
O’Leary ND, O’Connor KE, Duetz W, Dobson ADW (2001) Transcriptional regulation of styrene degradation in Pseudomonas putida CA-3. Microbiology 147:973–979
Otto K, Hofstetter K, Röthlisberger M, Witholt B, Schmid A (2004) Biochemical characterization of StyAB from Pseudomonas sp. strain VLB120 as a two-component flavin-diffusible monooxygenase. J Bacteriol 186:5292–5302. doi:10.1128/JB.186.16.5292-5302.2004
Panke S, Witholt B, Schmid A, Wubbolts MG (1998) Towards a biocatalyst for (S)-styrene oxide production: characterization of the styrene degradation pathway of Pseudomonas sp. strain VLB120. Appl Environ Microbiol 64:2032–2043
Parry RJ, Li W (1997) An NADPH:FAD oxidoreductase from the valanimycin producer, Streptomyces viridifaciens. Cloning, analysis, and overexpression. J Biol Chem 272:23303–23311. doi:10.1074/jbc.272.37.23303
Prieto MA, Garcia JL (1994) Molecular characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli. A two-protein component enzyme. J Biol Chem 269:22823–22829
Riedel A, Mehnert M, Heine T, Rathsack P, Kaschabek SR, Schlömann M, Tischler D (2013) GDCh - Wissenschaftsforum Chemie 2013, BIO 011. Darmstadt
Rudolph FB (1979) Product inhibition and abortive complex formation. Methods Enzymol 63:411–436
Russell TR, Tu S-C (2004) Aminobacter aminovorans NADH:flavin oxidoreductase His140: a highly conserved residue critical for NADH binding and utilization. Biochemistry 43:12887–12893. doi:10.1021/bi048499n
Russell TR, Demeler B, Tu S-C (2004) Kinetic mechanism and quaternary structure of Aminobacter aminovorans NADH:flavin oxidoreductase: an unusual flavin reductase with bound flavin. Biochemistry 43:1580–1590. doi:10.1021/bi035578a
Saa L, Jaureguibeitia A, Largo E, Llama MJ, Serra JL (2010) Cloning, purification and characterization of two components of phenol hydroxylase from Rhodococcus erythropolis UPV-1. Appl Microbiol Biotechnol 86:201–211. doi:10.1007/s00253-009-2251-x
Sambrook J, Fritsch EF, Maniatis T (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Plainview
Takeo M, Yasukawa T, Abe Y, Niihara S, Maeda Y, Negoro S (2008) Mechanism of 4-nitrophenol oxidation in Rhodococcus sp. Strain PN1: characterization of the two-component 4-nitrophenol hydroxylase and regulation of its expression. J Bacteriol 190:7367–7374. doi:10.1128/JB.00742-08
Thiel M, Kaschabek S, Gröning J, Mau M, Schlömann M (2005) Two unusual chlorocatechol catabolic gene clusters in Sphingomonas sp. TFD44. Arch Microbiol 183:80–94. doi:10.1007/s00203-004-0748-3
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. doi:10.1093/nar/25.24.4876
Thotsaporn K, Sucharitakul J, Wongratana J, Suadee C, Chaiyen P (2004) Cloning and expression of p-hydroxyphenylacetate 3-hydroxylase from Acinetobacter baumannii: evidence of the divergence of enzymes in the class of two-protein component aromatic hydroxylases. Biochim Biophys Acta 1680:60–66. doi:10.1016/j.bbaexp.2004.08.003
Tischler D, Kaschabek SR (2012) Microbial degradation of xenobiotics. In: Singh SN (ed) Environmental science and engineering. Springer, Heidelberg, pp 67–99
Tischler D, Eulberg D, Lakner S, Kaschabek SR, van Berkel WJH, Schlömann M (2009) Identification of a novel self-sufficient styrene monooxygenase from Rhodococcus opacus 1CP. J Bacteriol 191:4996–5009. doi:10.1128/JB.00307-09
Tischler D, Kermer R, Gröning JAD, Kaschabek SR, van Berkel WJH, Schlömann M (2010) StyA1 and StyA2B from Rhodococcus opacus 1CP: a multifunctional styrene monooxygenase system. J Bacteriol 192:5220–5227. doi:10.1128/JB.00723-10
Tischler D, Gröning JAD, Kaschabek SR, Schlömann M (2012) One-component styrene monooxygenases: an evolutionary view on a rare class of flavoproteins. Appl Biochem Biotechnol 167:931–944. doi:10.1007/s12010-012-9659-y
Tiwari MK, Singh RK, Lee J-K, Zhao H (2012) Mechanistic studies on the flavin:NADH reductase (PrnF) from Pseudomonas fluorescens involved in arylamine oxygenation. Bioorg Med Chem Lett 22:1344–1347. doi:10.1016/j.bmcl.2011.12.078
Toda H, Itoh N (2012) Isolation and characterization of styrene metabolism genes from styrene-assimilating soil bacteria Rhodococcus sp. ST-5 and ST-10. J Biosci Bioeng 113:12–19. doi:10.1016/j.jbiosc.2011.08.028
Tu S-C (2001) Reduced flavin: donor and acceptor enzymes and mechanisms of channeling. Antioxid Redox Sign 3:881–897. doi:10.1089/15230860152665046
Ukaegbu UE, Kantz A, Beaton M, Gassner GT, Rosenzweig AC (2010) Structure and ligand binding properties of the epoxidase component of styrene monooxygenase. Biochemistry 49:1678–1688. doi:10.1021/bi901693u
van Berkel WJH, Kamerbeek NM, Fraaije MW (2006) Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J Biotechnol 124:670–689. doi:10.1016/j.jbiotec.2006.03.044
van den Heuvel RHH, Westphal AH, Heck AJR, Walsh MA, Rovida S, van Berkel WJH, Mattevi A (2004) Structural studies on flavin reductase PheA2 reveal binding of NAD in an unusual folded conformation and support novel mechanism of action. J Biol Chem 279:12860–12867. doi:10.1074/jbc.M313765200
van Hellemond EW, van Dijk M, Heuts DPHM, Janssen DB, Fraaije MW (2008) Discovery and characterization of a putrescine oxidase from Rhodococcus erythropolis NCIMB 11540. Appl Microbiol Biotechnol 78:455–463. doi:10.1007/s00253-007-1310-4
van Lanen SG, Lin S, Horsman GP, Shen B (2009) Characterization of SgcE6, the flavin reductase component supporting FAD-dependent halogenation and hydroxylation in the biosynthesis of the enediyne antitumor antibiotic C-1027. FEMS Microbiol Lett 300:237–241. doi:10.1111/j.1574-6968.2009.01802.x
Velasco A, Alonso S, García JL, Perera J, Díaz E (1998) Genetic and functional analysis of the styrene catabolic cluster of Pseudomonas sp. strain Y2. J Bacteriol 180:1063–1071
Acknowledgments
The project was financed by the Saxon Ministry for Environment, Agriculture, and Geology (LfULG-1771508003). Dirk Tischler was supported by the European Social Fund and the Saxon Government (GETGEOWEB: 100101363). We are grateful to Adrie Westphal for advice during fluorescence measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gröning, J.A.D., Kaschabek, S.R., Schlömann, M. et al. A mechanistic study on SMOB-ADP1: an NADH:flavin oxidoreductase of the two-component styrene monooxygenase of Acinetobacter baylyi ADP1. Arch Microbiol 196, 829–845 (2014). https://doi.org/10.1007/s00203-014-1022-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-014-1022-y