Abstract
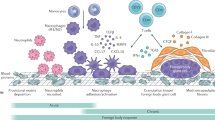
The use of polypropylene (PP) mesh for pelvic floor repair has been increasing dramatically over the past decade; however, tissue response in humans has not been extensively studied. This review discusses PP mesh and postimplantation host tissue response. Emphasis is placed on studies investigating the relationship between individual mesh properties and specific responses. There is an immediate inflammatory response after PP mesh implantation that lays the framework for tissue ingrowth and subsequent mesh integration. This response varies based on physical properties of individual mesh, such as pore size, weight, coatings, bacterial colonization, and biofilm production.


Similar content being viewed by others
References
Maher C, Feiner B, Baessler K, Glazener CMA (2010) Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews, issue 4
U. S. Food and Drug Administration. FDA Safety Communication: UPDATE on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse. July 2011. From: http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm262435.htm
Surgical repair of vaginal wall prolapse using mesh. National Institute for Health and Clinical Excellence. June 2008. From: http://guidance.nice.org.uk/IPG267/Guidance/pdf/English
Treating vaginal wall prolapse with surgery using mesh. National Institute for Health and Clinical Excellence. June 2008. From: http://guidance.nice.org.uk/IPG267/PublicInfo/pdf/English
Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ (1996) Changes in metabolism of collagen in genitourinary prolapse. Lancet 347:1658–1661
Kerkhof MH, Hendriks L, Brolmann HA (2009) Changes in connective tissue in patients with pelvic organ prolapse-a review of the current literature. Int Urogynecol J Pelvic Floor Dysfunct 20:461–474
Krause HG, Galloway SJ, Khoo SK, Lourie R, Goh JTW (2006) Biocompatible properties of surgical mesh using an animal model. Aust N Z J Obstet Gynecol 46:42–45
Amid PK (1997) Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1:15–21
Cosson M, Debodinance P, Boukerrou M et al (2003) Mechanical properties of synthetic implants used in the repair of prolapse and urinary incontinence in women: which is the ideal material? Int Urogynecol J 14:169–178
Junge K, Rosch R, Anurov M et al (2006) Modification of collagen formation using supplemented mesh materials. Hernia 10:492–497
Thiel M, Rodrigues Palma PC, Riccetto CL, Dambros M, Netto NR Jr (2005) A sterological analysis of fibrosis and inflammatory reaction induced by four different synthetic slings. BJU Int 95:833–837
de Almeida SHM, Rodrigues MA, Gregório E, Crespígio J, Moreira HA (2007) Influence of sling material on inflammation and collagen deposit in an animal model. Int J Uro 14:1040–1043
Junge K, Klinge U, Klosterhalfen B et al (2002) Influence of mesh materials on collagen deposition in a rat model. J Invest Surg 15:319–328
Pascual G, Rodriguez M, Gomez-Gil V, García-Honduvilla N, Buján J, Bellón JM (2008) Early tissue incorporation and collagen deposition in lightweight polypropylene meshes: bioassay in an experimental model of ventral hernia. Surgery 144:427–35
Anderson JM, Rodriguez A, Chang D (2008) Foreign body reaction to biomaterials. Semin Immunol 20(2):86–100
Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ (2005) Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng 11(1–2):1–18
Gorbet MB, Sefton MV (2004) Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 25(26):5681–703
Tang L, Jennings TA, Eaton JW (1998) Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc Natl Acad Sci 95(15):8841–6
Xia Z, Triffitt JT (2006) A review on macrophage responses to biomaterials. Biomed Mater 1:R1–R9
Collier TO, Anderson JM, Kikuchi A, Okano T (2002) Adhesion behavior of monocytes, macrophages, and foreign body giant cells on poly (N-isopropylacrylamide) temperature-responsive surfaces. J Biomed Mater Res 59:136–143
Brodbeck WG, Voskerician G, Ziats NP, Nakayama Y, Matsuda T, Anderson JM (2003) In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J Biomed Mater Res A 64(2):320–9
Di Vita G, Milano S, Patti R et al (2001) Cytokine modifications after tension-free hernioplasty or open conventional inguinal hernia repair. Am J Surg 181(6):487–491
Di Vita G, D’ Agostino P, Patti R et al (2005) Acute inflammatory response alter inguinal and incisional hernia repair with implantation of polypropylene mesh of different size. Langenbecks Arch Surg 390:301–311
Rechberger T, Jankiewicz K, Adamiak A, Miotla P, Chrobak A, Jerzak M (2009) Do preoperative cytokine levels offer a prognostic factor for polypropylene mesh erosion after suburethral sling surgery for stress urinary incontinence? Int Urogynecol J 20:69–74
Orenstein SB, Saberski ER, Klueh U (2010) Effects of mast cell modulation on early host response to implanted synthetic meshes. Hernia 14:511–516
Junge K, Binnebosel M, Rosch R et al (2009) Impact of proinflammatory cytokine knockout on mesh integration. J Invest Surg 22:256–262
Pierce LM, Rao A, Baumann SS, Glassberg JE, Kuehl TJ, Muir TW (2009) Long-term histologic response to synthetic and biologic graft materials implanted in the vagina and abdomen of a rabbit model. Am J Obstet Gynecol 546:e1–e8
Ott R, Hartwig T, Tannapfel A et al (2007) Biocompatibility of bacterial contaminated prosthetic meshes and porcine dermal collagen used to repair abdominal wall defects. Langenbecks Arch Surg 392:473–478
Culligan P, Heit M, Blackwell L, Murphy M, Graham CA, Snyder J (2003) Bacterial colony counts during vaginal surgery. Infect Dis Obstet Gynecol 11:161–5
Vollebregt A, Troelstra A, Huub van der Vaart C (2009) Bacterial colonization of collagen-coated polypropylene vaginal mesh: are additional intraoperative sterility procedures useful? Int Urogynecol J 20:1345–1351
Wang AC, Wu R, Lin C (2008) A microbiological and immunohistochemical analysis of periurethral and vaginal tissue in women with de novo urge symptoms after mid-urethral sling procedures-a prospective case-controlled study. Int Urogynecol J 19:1145–1150
Sternberg C, Christensen BB, Johansen T et al (1999) Distribution of bacterial growth activity in flow-chamber biofilms. Appl Environ Microbiol 65(9):4108–4117
Kathju S, Nistico L, Lasko L, Stoodley S (2010) Bacterial biofilm on monofilament suture and porcine xenograft after inguinal herniorrhaphy. FEMS Immunol Med Microbiol 59:405–409
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Suci PA, Mittelman MW, Yu FP, Geesey GG (1994) Investigation of ciprofloxacin penetration into pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 38(9):2125–2133
Bjarnsholt T, Jensen PO, Fiandaca MJ (2009) Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol 44:547–558
Hoyle BD, Costerton JW (1989) Transient exposure to physiologically-relevant concentration of calcium confers tobramycin resistance upon sessile cells of pseudomonas aeruginosa. FEMS Microbiol Let 60:339–342
Evans DJ, Brown MR, Allison DG, Gilbert P (1990) Susceptibility of bacterial biofilms to tobramycin: role of specific growth rate and phase in the division cycle. J Antimicro Chemotherap 25:585–591
Gao M, Han J, Tian J, Yang K (2010) Vypro II mesh for inguinal hernia repair. Ann Surg 251(5):838–842
Klinge U, Schumpelick V, Klosterhalfen B (2001) Functional assessment and tissue response of short and long term absorbable surgical meshes. Biomaterials 22:1415–1424
Rosch R, Junge K, Schachtrupp A, Klinge U, Klosterhalfen B, Schumpelick V (2003) Mesh implants in hernia repair. Eur Surg Res 35:161–165
Rosch R, Junge K, Quester R, Klinge U, Klosterhalfen B, Schumpelick V (2003) Vypro II mesh in hernia repair: impact of polyglactin on long term incorporation in rats. Eur Surg Res 35:445–450
Krause HG, Galloway SJ, Khoo SK, Lourie R, Goh JT (2006) Biocompatible properties of surgical mesh using an animal model. Aust N Z J Obstet Gynaecol 46:42–45
Junge K, Rosch R, Krones CJ et al (2005) Influence of polyglecaprone 25 (monocryl) supplementation on the biocompatibility of a polypropylene mesh for hernia repair. Hernia 9:212–217
Bellon JM, Garcia-Honduvilla N, Serrano N, Rodríguez M, Pascual G, Buján J (2005) Composite prostheses for the repair of abdominal wall defects: effect of the structure of the adhesion barrier component. Hernia 9:338–343
Papademetriou P, Petros P (2005) Histological studies of monofilament and multifilament polypropylene mesh implants demonstrate equivalent penetration of macrophages between fibrils. Hernia 9:75–78
Kumazawa R, Watari F, Takashi N, Tanimura Y, Uo M, Totsuka Y (2002) Effects of ti ions and particles on neutrophil function and morphology. Biomaterials 23:3757–3764
Biomet 2011. From: http://www.biomet.com/biologics/timesh.cfm
Scheidbach H, Tannapfel A, Schmidt U, Lippert H, Köckerling F (2004) Influence of titanium coating on the biocompatibility of a heavyweight polypropylene mesh. Eur Surg Res 36:313–317
Junge K, Rosch R, Klinge U et al (2005) Titanium coating of a propylene mesh for hernia repair: effect on biocompatibility. Hernia 9:115–119
Ku C, Browne M, Gregson PJ, Corbeil J, Pioletti DP (2002) Large scale gene expression analysis of osteoblasts cultured on three different Ti-6A1-4V surface treatments. Biomaterials 23:4193–4202
Carinci F, Volinia S, Pezzetti F, Francioso L, Tosi L, Piatelli A (2003) Titanium cell interaction: analysis of gene expression profiling. J Biomed Mater Res 66B:341–346
Parenteau-Bareil R, Gauvin R, Berthod F (2010) Collagen based biomaterials for tissue engineering applications. Mater 3:1863–1887
Sergent F, Resch B, Al-khattabi M, Ricbourg A, Schaal JP, Marpeau L (2011) Transvaginal mesh repair of pelvic organ prolapse by the transobturator-infracoccygeal hammock technique: long term anatomical and functional outcomes. Neurourol Urodynam 30:384–389
Huffaker RK, Muir TW, Rao A, Baumann SS, Kuehl TJ, Pierce LM (2008) Histologic response of porcine collagen-coated and uncoated polypropylene grafts in a rabbit vagina model. Am J Obstet Gynecol 198:582.e1–582.e7
De Tayrac R, Alves A, Therin M (2007) Collagen-coated vs. noncoated low-weight polypropylene meshes in a sheep model for vaginal surgery. A pilot study. Int Urogynecol J 18:513–520
Pierce LM, Asarias JR, Nguyen PT, Mings J, Gehrich A (2011) Inflammatory cytokine and matrix metalloproteinase expression induced by collagen-coated and uncoated polypropylene meshes in a rat model. Am J Obstet Gynecol 205:82.e1–9
van Luyn MJA, van Wachem PB, Olde Damink LHH, Dijkstra PJ, Feijen J, Nieuwenhuis P (1992) Relations between in vitro cytotoxicity and crosslinked dermal sheep collagens. J Biomed Mater Res 26:1091–1110
Chu CC, Welch L (1985) Characterization of morphological and mechanical properties of surgical mesh fabrics. J Biomed Mater Res 19:903–916
Pourdeyhimi B (1989) Porosity of surgical mesh fabric: new technology. J Biomed Mater Res 23:145–152
Bazi TM, Hamade RF, Hussein IAH, Nader KA, Jurjus A (2007) Polypropylene midurethral tapes do not have similar biologic and biomechanical performance in the rat. Eur Urol 51:1364–1375
Dora CD, Dimarco DS, Zobitz ME, Elliott DS (2004) Time dependent variations in biomechanical properties of cadaveric fascia, porcine dermis, porcine small intestine submucosa, polypropylene mesh and autologous fascia in the Rabbit model: implications for sling surgery. J Urol 171:1970–1973
Bringman S, Conze J, Cuccurullo D et al (2010) Hernia repair: the search for ideal meshes. Hernia 14:81–87
Agarwal BB, Agarwal KA, Mahajan KC (2009) Prospective double-blind randomized controlled study comparing heavy and lightweight polypropylene mesh in totally extraperitoneal repair of inguinal hernia: early results. Surg Endosc 23:242–247
Horstmann R, Hellwig M, Classen C, Röttgermann S, Palmes D (2006) Impact of polypropylene amount on functional outcome and quality of life after inguinal hernia repair by the TAPP procedure using pure, mixed, and titanium coated meshes. World J Surg 30:1–8
Klosterhalfen B, Junge K, Klinge U (2005) The lightweight and large porous mesh concept for hernia repair. Expert Rev Med Devices 2(1):103–117
Deffieux X, Tayrac R, Huel C et al (2007) Vaginal mesh erosion after transvaginal repair of cystocele using Gynemesh or Gynemesh-Soft in 138 women: a comparative study. Int Urogynecol J 18:73–79
Klinge U, Junge K, Stumpf M, Öttinger AP, Klosterhalfen B (2002) Functional and morphological evaluation of a low-weight, monofilament polypropylene mesh for hernia repair. J Biomed Mater Res 63:129–136
Novitsky YW, Cristiano JA, Harrell AG et al (2008) Immunohistochemical analysis of host reaction to heavyweight, reduced weight, and expanded polytetrafluoroethylene (ePTFE)-based meshes after short and long term intraabdominal implantations. Surg Endosc 22:1070–1076
Klinge U, Junge K, Spellerberg B, Piroth C, Klosterhalfen B, Schumpelick V (2002) Do multifilament alloplastic meshes increase the infection rate? Analysis of the polymeric surface, the bacteria adherence, and the in vivo consequences in a rat model. J Biomed Mater Res 63:765–771
Taylor DF, Smith FB (1972) Porous methyl methacrylate as an implant material. J Biomed Mater Res Symp 2(2):467–479
Chvapil M, Holuša R, Kliment K, Štoll M (1969) Some chemical and biological characteristics of a new collagen-polymer compound material. J Biomed Mater Res 3:315–332
Bobyn JD, Wilson GJ, MacGregor DC, Pillar RM, Weatherly GC (1982) Effect of pore size on the peel strength of attachment of fibrous tissue to porous-surfaced implants. J Biomed Mater Res 16:571–584
Greca FH, de Paula JB, Biondo-Simoes MLP et al (2001) The influence of differing pore sizes on the biocompatibility of two polypropylene meshes in the repair of abdominal defects. Hernia 5:59–64
Greca FH, Souza-Filho ZA, Giovanini A et al (2008) The influence of porosity on the integration histology of two polypropylene meshes for the treatment of abdominal wall defects in dogs. Hernia 12:45–49
Williams DF (1982) Review biodegradation of surgical polymers. J Mater Sci 17:1233–1246
Jongebloed WL, Worst JF (1986) Degradation of polypropylene in the human eye: a sem-study. Docu Ophthal 64:143–152
Coda A, Bendavid R, Botto-Micca F (2003) Structural alterations of prosthetic meshes in humans. Hernia 7:29–34
Costello CR, Bachman SL, Grant SA (2007) Characterization of heavyweight and lightweight polypropylene prosthetic mesh explants from a single patient. Surg Innov 14(3):168–176
Costello CR, Bachman SL, Grant SA (2007) Materials characterization of explanted polypropylene hernia meshes. J Biomed Mater Res Part B: Appl Biomater 83B:44–49
Frostling H, Hoff A, Jacobsson S, Pfäffli P, Vainiotalo S, Zitting A (1984) Analytical, occupational and toxicologic aspects of the degradation products of polypropylene plastics. Scan J Work Environ Heal 10:163–169
Conflicts of interest
Patel: None; Ostergard: American Medical Systems, consultant; Sternschuss: None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, H., Ostergard, D.R. & Sternschuss, G. Polypropylene mesh and the host response. Int Urogynecol J 23, 669–679 (2012). https://doi.org/10.1007/s00192-012-1718-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1718-y