Abstract
Purpose
Weaning-induced cardiac dysfunction is more likely to occur if the heart does not tolerate the changes in loading conditions induced by spontaneous breathing trial (SBT). We hypothesized that the presence of cardiac preload independence before an SBT is associated with weaning failure related to cardiac dysfunction.
Methods
We included 30 patients after a first failed 1-h T-tube SBT who had a transpulmonary thermodilution already in place. Preload independence [no increase in the pulse contour analysis-derived cardiac index ≥10 % during passive leg raising (PLR)] was assessed before the second SBT. Failure of the SBT related to cardiac dysfunction was defined by an increase in pulmonary artery occlusion pressure above 18 mmHg at the end of the SBT associated with clinical intolerance.
Results
Fifty-seven SBTs were analyzed. The SBT failed in 46 cases. Overall, 31 failed SBTs were associated with weaning-induced cardiac dysfunction. During PLR, the cardiac index did not change in cases of failed SBTs with cardiac dysfunction, whereas it significantly increased in the other cases: 4 % (interquartile range, IQR 0–5) vs. 12 % (IQR 11–15), respectively. If PLR did not increase the cardiac index by more than 10 % before the SBT, the occurrence of SBT failure related to cardiac dysfunction was predicted with a sensitivity of 97 % [95 % confidence interval (CI) 83–100], specificity of 81 % (95 % CI 61–93) and area under the receiver-operating characteristic curve of 0.88 (95 % CI 0.78–0.98).
Conclusions
Preload independence assessed by a negative PLR test performed before an SBT predicts weaning failure related to cardiac dysfunction.
Similar content being viewed by others
Introduction
Weaning-induced cardiac dysfunction is one of the most frequent causes involved in cases of failure of a spontaneous breathing trial (SBT) [1]. The main mechanisms that lead to cardiac dysfunction and hence pulmonary edema during weaning include an increase in cardiac preload and/or an increase in LV afterload and/or a potential increase in the right ventricular (RV) afterload [1]. Weaning-induced cardiac dysfunction may occur in cases where the heart is unable to tolerate the SBT-induced changes in loading conditions [1]. It is likely that this phenomenon occurs more often in the case of cardiac preload independence. To test this hypothesis, we undertook this study to investigate the cardiac preload status (dependence or independence) in patients who failed a first SBT. To determine the cardiac preload status, we used passive leg raising (PLR), a dynamic test already described to detect preload dependence in the context of shock states [2]. By transferring a patient from the semirecumbent position to supine, PLR transfers venous blood from the legs and abdominal compartment toward the intrathoracic compartment and increases cardiac output in case of preload dependence [2]. Determining cardiac preload status can thus be performed by measuring the real-time changes in cardiac output during a PLR test. Several methods are currently available for this purpose, such as echocardiography [3, 4], invasive or noninvasive pulse contour analysis [5–8]. In the present study, we used the pulse contour analysis calibrated by transpulmonary thermodilution.
We hypothesized that a preload independence status during mechanical ventilation, indicated by the absence of an increase in cardiac output during a PLR test (negative PLR), is more likely associated with weaning-induced cardiac dysfunction and that this weaning-induced cardiac dysfunction could be associated with weaning failure.
Preliminary results of the present study have been presented at the ESCIM LIVES Congress 2013 [9].
Materials and methods
Patients
The present study was conducted between June 2012 and September 2013. As approved by the institutional review board of our institution (Comité Pour la Protection des Personnes Ile-de-France 7), all patients were informed about the study and accepted to participate. They must have failed a first 1-h SBT, whatever the cause of intubation and underlying pulmonary disease. They also must have had a transpulmonary thermodilution device already in place (PiCCO2 device, Pulsion Medical Systems, Munich, Germany). This device had been set up at the early phase of resuscitation, and the arterial catheter for transpulmonary thermodilution had not been removed at the time of weaning. The screening criteria for performing an SBT were those of current guidelines [10]. The only exclusion criteria were neuromuscular disease, tracheostomy and a contraindication to a pulmonary artery catheter.
Study design
According to our usual practice in such a particular category of patients, a pulmonary artery catheter (CCOmboV, Edwards Lifesciences, Irvine, CA) was inserted after a first failed SBT. A second SBT was performed the next day. Just before starting the SBT, we recorded the respiratory rate, heart rate, systemic and pulmonary artery pressures and pulmonary artery occlusion pressure (PAOP). All pressures were measured at end-expiration. All PAOP tracings were reviewed offline by an investigator (XM) who did not perform the measurement at the time of SBT. We also performed transpulmonary thermodilution measurements and recorded the cardiac index, global end-diastolic volume and extravascular lung water. The values of three successive transpulmonary thermodilution measurements were averaged [11]. The LV ejection fraction was measured with transthoracic echocardiography (CX50, Philips Healthcare, Andover, CA) by two investigators (MD and XM) using the biplane or monoplane Simpson method. Significant valvulopathies were defined as grade 2 or more aortic and mitral regurgitations and moderate-to-severe aortic and mitral stenosis.
The investigators (MD and LG) then performed a PLR test according to the previously described modalities [12]. This was done by transferring the patient from the semirecumbent position to a position with the legs raised at 45° and horizontal trunk. It was performed by moving the patient’s bed. The pressure transducer was attached to the patient's arm in order to keep the height between it and the cardiac chambers constant. The maximal changes in the pulse contour analysis-derived cardiac index (PiCCO2 device) during PLR were recorded. They usually occur in a few seconds, always within 1 min [2]. The patient was then moved back to the semirecumbent position. The SBT was then performed by connecting the patient to a T-piece. The SBT was interrupted at 60 min or earlier if patients exhibited any signs of intolerance among the following: (1) diaphoresis, (2) use of accessory respiratory muscles, (3) worsening of discomfort, (4) respiratory rate ≥35 breaths per minute, (5) pulse oxygen saturation ≤90 %, (6) heart rate ≥140 beats per minute and/or (7) systolic arterial pressure ≥180 mmHg. The decision to stop the SBT was made by the attending physician, who was not involved in the study. Before reconnecting the patient to the ventilator, the investigators recorded the respiratory rate, heart rate, systemic arterial pressure, pulmonary artery pressure, PAOP, cardiac index (transpulmonary thermodilution), extravascular lung water and global end-diastolic volume. Failed SBT due to weaning-induced cardiac dysfunction was diagnosed if patients exhibited signs of intolerance associated with a PAOP value above 18 mmHg at the end of the SBT (at 60 min or earlier in case of intolerance) [13]. Clinical intolerance was defined as an increase in the respiratory rate above 35 per minute, desaturation with pulse oxygen saturation <90 %, use of the accessory respiratory muscles and hypertension with systolic arterial pressure above 180 mmHg. If the patient failed at an SBT, we repeated the SBT during the next days until the patient was extubated. These ensuing SBTs were included in the analysis if the pulmonary artery catheter and transpulmonary thermodilution device were still in place. The management of patients with a failed SBT was left to the discretion of the clinicians in charge. In particular, the diagnosis of a failed SBT due to cardiac dysfunction could have led to fluid removal (diuretics or ultrafiltration) and/or nitrate administration.
Statistical analysis
The normality of data was tested by the Kolmogorov-Smirnov normality test. Continuous variables were expressed as median (interquartile range, IQR). Taking into account a mean cardiac index of 3.2 and considering that PLR was positive if the cardiac index increased by more than 10 %, with an alpha-risk of 5 % and a beta-risk of 20 %, we calculated that 30 patients needed to be included in the study. Comparisons between before vs. during PLR and between before vs. the end of the SBT were assessed with a Wilcoxon test. Comparisons of variables between cases with vs. cases without weaning-induced cardiac dysfunction were assessed by a two-tailed Student’s t test or a Mann-Whitney U test, as appropriate. A receiver-operating characteristic (ROC) curve was constructed to test the ability of the PLR-induced changes in the cardiac index to predict a weaning-induced cardiac dysfunction. Sensitivities, specificities and areas under (AUCs) the ROC curve are expressed as mean (95 % CI). The diagnostic cutoff was determined by the best Youden index value. Since some patients underwent several SBTs, each SBT was considered as a “case,” and all cases were included in the primary analysis. We also performed a secondary analysis in which we analyzed only one SBT per patient, which was the first performed just after inclusion. The statistical analysis was performed with the GraphPad software (Prism 6.0b, Macintosh version, by Software MacKiev).
Results
Patients
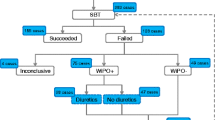
During the study period, 1,350 patients were hospitalised in our unit. Among them, 450 were mechanically ventilated for more than 24 h and 320 had a transpulmonary thermodilution device. One hundred twenty-six patients had a PiCCO catheter in place at the time of the weaning process. Among them, 35 failed their first SBT. Eventually, 30 patients were included in the study. Their main characteristics are described in Table 1. The LV ejection fraction at baseline was not different between the two groups. A total of 57 SBTs were performed (Fig. 1). The SBT failed in 46 cases. Among them, weaning-induced cardiac dysfunction was detected in 31 cases. Overall, 26 SBTs were not associated with weaning-induced cardiac dysfunction. Eleven SBTs were successful and were followed by extubation. The outcomes of the SBTs according to the response to PLR are displayed in Figure ESM1.
Hemodynamic effects of SBT: association between preload independence at baseline and the occurrence of weaning-induced cardiac dysfunction
The hemodynamic effects of SBTs are presented in Table 2. In cases with weaning-induced cardiac dysfunction, the PAOP increased by 12 mmHg (interquartile range, IQR 10–17) (Table 2).
PLR was not associated with any adverse effect. It increased the cardiac index to a smaller extent in cases with a weaning-induced cardiac dysfunction compared to cases without: 4 % (IQR 0–5) vs. 12 % (IQR 11–15), respectively, p < 0.0001 (Table 2 and Fig. 2).
Detection of preload dependence in the 57 spontaneous breathing trials (primary analysis). Box and whiskers representing the changes in the cardiac index induced by passive leg raising performed before the spontaneous breathing trial; *p < 0.05. Light gray box: cases with weaning-induced cardiac dysfunction, dark gray box: cases without weaning-induced cardiac dysfunction
If the cardiac index did not increase by more than 10 % during PLR (indicating preload independence [14]), the occurrence of weaning-induced cardiac dysfunction could be predicted with a sensitivity of 97 % (95 % CI 83–100) and specificity of 81 % (95 % CI 61–93) (Figure ESM2). The AUC of the ROC curve was 0.88 (95 % CI 0.78–0.98) (Figure ESM2). The positive and negative predictive values were respectively 0.86 and 0.87, the Youden index was 0.78, and the likelihood ratio was 5. The AUC of the ROC curve for PAOP at baseline was 0.58 (95 % CI 0.44–0.73).
Four patients have been followed during successive SBTs until they did not anymore present weaning-induced cardiac dysfunction (Table ESM1). All these patients had negative PLR tests associated with weaning-induced cardiac dysfunction. Diuretics were administered. The following SBTs were no longer associated with weaning-induced cardiac dysfunction when the PLR test became positive.
Analysis restricted to the first SBT of each patient
The secondary analysis restricted to the 30 first SBTs performed after inclusion provided results similar to the primary analysis. In patients with weaning-induced cardiac dysfunction, the PAOP increased by 11 mmHg (IQR 10–17). PLR increased the cardiac index to a smaller extent in patients with a weaning-induced cardiac dysfunction compared to patients without [3 % (IQR 0–5) vs. 12 % (IQR 8–15), respectively, p < 0.01].
If the cardiac index did not increase by more than 10 % during PLR (indicating preload independence), the occurrence of weaning-induced cardiac dysfunction could be predicted with a sensitivity of 100 % (95 % CI 80–100) and a specificity of 71 % (95 % CI 42–92). The AUC of the ROC curve was 0.86 (95 % CI 0.71–1.01). The positive and negative predictive values were respectively 0.82 and 1.0, the Youden index was 0.71, and the likelihood ratio was 4.
Analysis restricted to the 46 failed spontaneous breathing trials
The analysis restricted to the 46 failed SBTs showed that in cases with weaning-induced cardiac dysfunction (n = 29), the PAOP increased by 13 mmHg (IQR 10–19). PLR increased the cardiac index to a smaller extent in cases with a weaning-induced cardiac dysfunction compared to patients without [4 % (IQR 0–5) vs. 12 % (IQR 7–14), respectively, p < 0.01].
If the cardiac index did not increase by more than 10 % during PLR, the occurrence of weaning-induced cardiac dysfunction could be predicted with a sensitivity of 97 % (95 % CI 82–100) and specificity of 65 % (95 % CI 38–86). The AUC of the ROC curve was 0.82 (95 % CI 0.68–0.97). The positive and negative predictive values were respectively 0.83 and 0.97, the Youden index was 0.62, and the likelihood ratio was 3.
Discussion
The present study confirmed our hypothesis that a negative PLR test performed before an SBT, indicating preload independence, can reliably identify failure of the SBT associated with weaning-induced cardiac dysfunction.
PLR induces a translocation of venous blood from the legs and the splanchnic compartment toward the cardiac chambers [2]. In fact, it increases the mean systemic pressure by means of the hydrostatic pressure created by leg elevation. Eventually, PLR increases the right and left ventricular preload. The effects of PLR on cardiac output depend on the preload reserve of both ventricles. If the right ventricle is preload responsive, an increase in its preload results in an increase in right cardiac output and hence in left ventricular filling. If the left ventricle is also preload dependent, this increase in its preload eventually induces an increase in stroke volume [2]. A negative PLR test could indicate preload independence of either the right or left ventricle, or of both. Such conditions can be associated with several conditions that all lead to weaning-induced cardiac dysfunction. First, in case of preload independence of the right ventricle, the increases in venous return and RV afterload during the SBT—as suggested in our study by the increase in pulmonary artery pressure—are likely to result in a further RV dilation. This could eventually lead to a right-to-left shift of the interventricular septum, impeding LV filling, and hence to an increase in LVEDP. Second, preload independence of the left ventricle is likely to be associated with LV failure. In this condition, a further SBT-induced increase in the LV preload (volume) may result in a huge increase in LVEDP. Furthermore, in this condition of LV preload independence, the left ventricle is more likely to be sensitive to increases in its afterload. The SBT-induced increase in LV afterload is thus more likely to increase the LVEDP. In line with this, a limitation of our study was that we did not assess the RV systolic function in order to correlate it with the presence of RV preload independence. One must nevertheless admit that such an assessment is difficult because of the crescent shape of the right ventricle even with the current echocardiographic approach.
Interestingly, on average the LV ejection fraction at baseline was normal in patients in both groups. A normal LV ejection fraction is likely to be associated with LV preload dependence. This suggests that preload independence of the right ventricle without preload independence of the left ventricle could be sufficient to explain the occurrence of weaning-induced cardiac dysfunction in some patients. In this regard, an advantage of the PLR test could be to test both RV and LV preload independence. This also confirms that assessment of the LV ejection fraction alone before performing an SBT is not able to identify all patients who will present a weaning-induced cardiac dysfunction. This latter point was already reported by previous studies [15–18].
In agreement with our results, it has been reported that respiratory failure in patients presenting with it at the emergency department is more likely of cardiac origin if a postural change with lowering of the trunk to a horizontal position is not associated with an increase in the cardiac index [19]. These results were nevertheless not confirmed in another study [20] that used PLR as the postural change. However, in these studies, the postural changes were assessed by bioreactance, a technique whose reliability in assessing the effects of the PLR test is debated [21, 22].
Cardiac dysfunction is increasingly recognized as an important cause of weaning failure [23]. The incidence of weaning-induced cardiac dysfunction as a cause of weaning failure ranges from 20 % [24, 25] in some studies to more than 40 % [16, 18, 26, 27] in other ones. Such differences might be explained by the fact that various methods have been used to detect weaning-induced cardiac dysfunction [28]. We inserted a pulmonary artery catheter to diagnose weaning-induced cardiac dysfunction, which could be judged somehow fulsome. It must be underlined that patients in whom we did this were indirectly selected because of the severity of their condition. Indeed, they were still equipped with a transpulmonary thermodilution device, which had been required for managing their severe acute illness. Nowadays, clinicians have several tools to diagnose weaning-induced cardiac dysfunction. Some of them are noninvasive, such as detecting an increase in the plasma protein concentration [18, 26] and plasma B-type natriuretic peptides [29], or echocardiography [16, 17, 24]. The benefit of the present method is to provide physicians with additional information before performing the SBT.
Importantly, the baseline PAOP value did not provide a reliable prediction of weaning-induced cardiac dysfunction. This has been confirmed by many previous results [13, 16, 26, 30]. A dynamic approach using PLR is thus better than an approach based on static measurements of the cardiac preload. In this regard, a previous study by our group showed that ratios of the E over A or E over E’ waves of the mitral flow and annulus velocity measured before the SBT did not predict weaning-induced cardiac dysfunction [16].
Our results do not imply that PLR should replace SBT before deciding to extubate the patient, since PLR is obviously not able to detect weaning failure of non-cardiac origin. We rather believe that the combination of a PLR test and SBT could guide therapeutic options in patients who fail an SBT. Indeed, the treatment of weaning-induced cardiac dysfunction usually includes diuretic administration before the next SBT and nitrate administration during the next SBT. Even though fluid removal can be effective in case of suspicion of fluid overload [28, 31], this therapeutic option is (1) not without risks and (2) is too frequently used even when the cause of weaning failure has not been elucidated. Our results suggest that, after a failed SBT that is preceded by a positive PLR test, fluid removal should not be the preferred therapeutic option. Conversely, in case of weaning failure associated with a negative PLR, fluid removal should be a therapeutic option to initiate first and to continue until the PLR test becomes positive. It should not impede nitrate administration during the next SBT, especially in case of systemic hypertension. In line with this, we observed that in some patients who failed the SBT because of weaning-induced cardiac dysfunction, cardiac-related weaning failure did not re-occur once the PLR became positive. The present study was not designed to investigate the impact of a preload dependence-directed therapy in the setting of weaning failure. Whether such a strategy could make the weaning process faster, as has been demonstrated for the BNP [32], should now be investigated.
Of note, we included all the SBTs performed by the 30 patients (n = 57) in the primary analysis, including successful and failed SBTs. We believe that detecting cardiac dysfunction in patients who succeed could be of some potential interest. This should motivate physicians to carefully explore cardiac function afterwards and to suspect a cardiac dysfunction if a respiratory failure occurs in the next days following weaning.
A limitation of using PLR in the context of weaning is that it requires a technique to measure cardiac output. In our study, we used the pulse contour method calibrated with transpulmonary thermodilution because the device was already in place. However, we do not recommend using such an invasive device only for the purpose of guiding weaning. Some less invasive methods, such as echocardiography or noninvasive arterial pulse contour analysis, are preferable in this context.
Our study suffers from several limitations. First, we only used a T-piece trial to perform the SBT, but SBTs with pressure support induce less extreme deleterious heart-lung interactions [33]. However, our choice should have amplified but not altered our physiological findings. Second, some patients were included several times in the study analysis. This helped us to identify patients in whom the SBT eventually became successful while the PLR test eventually became positive, a result that strengthens our message. Nevertheless, restricting the analysis to the first SBT in each patient provided similar results to those obtained when all cases were taken into account. Third, although myocardial ischemia could theoretically account for the weaning-induced cardiac dysfunction [34], we did not assess it by electrocardiogram or troponin measurements. Nevertheless, we found only one patient exhibiting weaning-induced cardiac dysfunction in spite of a positive PLR test. This suggests that this limitation did not influence our results to a large extent. Fourth, our practice to use a pulmonary artery catheter to diagnose weaning-induced cardiac dysfunction could be questioned. Nevertheless, even though some alternative techniques have been described, the PAOP measurement is still the reference method for diagnosing weaning-induced cardiac dysfunction. Finally, we did not specifically investigate some other conditions that could be associated with weaning-induced cardiac dysfunction, such as hypertrophic obstructive cardiomyopathy [35].
In conclusion, a negative PLR test performed before a failing SBT, suggesting the presence of preload independence, was associated with weaning-induced cardiac dysfunction. Conversely, our data suggest that a positive PLR test can rule out a cardiac origin of weaning failure. Whether the PLR test can guide treatment of weaning failure of cardiac origin should be further investigated.
References
Teboul J-L (2014) Weaning-induced cardiac dysfunction: where are we today? Intensive Care Med 40:1069–1079
Monnet X, Teboul JL (2008) Passive leg raising. Intensive Care Med 34:659–663
Lamia B, Ochagavia A, Monnet X et al (2007) Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med 33:1125–1132
Maizel J, Airapetian N, Lorne E et al (2007) Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med 33:1133–1138
Monnet X, Bleibtreu A, Ferré A et al (2012) Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med 40:152–157
Monnet X, Dres M, Ferré A et al (2012) Prediction of fluid responsiveness by a continuous non-invasive assessment of arterial pressure in critically ill patients: comparison with four other dynamic indices. Br J Anaesth 109:330–338
Biais M, Nouette-Gaulain K, Roullet S et al (2009) A comparison of stroke volume variation measured by Vigileo/FloTrac system and aortic Doppler echocardiography. Anesth Analg 109:466–469
Marik PE, Monnet X, Teboul JL (2011) Hemodynamic parameters to guide fluid therapy. Ann Intensive Care 1:1
Dres M, Teboul J-L, Anguel N et al (2013) Preload independency as detected by passive leg raising is associated with weaning-induced pulmonary edema. In: Oral presentation at the ESCIM LIVES congress 2013
Boles J-M, Bion J, Connors A et al (2007) Weaning from mechanical ventilation. Eur Respir J 29:1033–1056
Monnet X, Persichini R, Ktari M et al (2011) Precision of the transpulmonary thermodilution measurements. Crit Care 15:R204
Jabot J, Teboul JL, Richard C, Monnet X (2009) Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med 35:85–90
Lemaire F, Teboul JL, Cinotti L et al (1988) Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology 69:171–179
Monnet X, Rienzo M, Osman D et al (2006) Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 34:1402–1407
Moschietto S, Doyen D, Grech L et al (2012) Transthoracic echocardiography with Doppler tissue imaging predicts weaning failure from mechanical ventilation: evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit Care 16:R81
Lamia B, Maizel J, Ochagavia A et al (2009) Echocardiographic diagnosis of pulmonary artery occlusion pressure elevation during weaning from mechanical ventilation. Crit Care Med 37:1696–1701
Papanikolaou J, Makris D, Saranteas T et al (2011) New insights into weaning from mechanical ventilation: left ventricular diastolic dysfunction is a key player. Intensive Care Med 37:1976–1985
Dres M, Teboul J-L, Anguel N et al (2014) Extravascular lung water, B-type natriuretic peptide, and blood volume contraction enable diagnosis of weaning-induced pulmonary edema. Crit Care Med 42:1882–1889
Engineer RS, Benoit JL, Hicks CW et al (2012) Hemodynamic changes as a diagnostic tool in acute heart failure—a pilot study. Am J Emerg Med 30:174–180
García X, Simon P, Guyette FX et al (2013) Noninvasive assessment of acute dyspnea in the ED. Chest 144:610–615
Marik PE, Levitov A, Young A, Andrews L (2013) The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest 143:364–370
Kupersztych-Hagege E, Teboul J-L, Artigas A et al (2013) Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth 111:961–966
Perren A, Brochard L (2013) Managing the apparent and hidden difficulties of weaning from mechanical ventilation. Intensive Care Med 39:1885–1895
Caille V, Amiel J-B, Charron C et al (2010) Echocardiography: a help in the weaning process. Crit Care 14:R120
Zapata L, Vera P, Roglan A et al (2011) B-type natriuretic peptides for prediction and diagnosis of weaning failure from cardiac origin. Intensive Care Med 37:477–485
Anguel N, Monnet X, Osman D et al (2008) Increase in plasma protein concentration for diagnosing weaning-induced pulmonary oedema. Intensive Care Med 34:1231–1238
Grasso S, Leone A, De Michele M et al (2007) Use of N-terminal pro-brain natriuretic peptide to detect acute cardiac dysfunction during weaning failure in difficult-to-wean patients with chronic obstructive pulmonary disease. Crit Care Med 35:96–105
Dres M, Teboul J-L, Monnet X (2014) Weaning the cardiac patient from mechanical ventilation. Curr Opin Crit Care 20:493–498
Mekontso-Dessap A, de Prost N, Girou E et al (2006) B-type natriuretic peptide and weaning from mechanical ventilation. Intensive Care Med 32:1529–1536
Jubran A, Mathru M, Dries D, Tobin MJ (1998) Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof. Am J Respir Crit Care Med 158:1763–1769
Thille AW, Richard J-CM, Brochard L (2013) The decision to extubate in the intensive care unit. Am J Respir Crit Care Med 187:1294–1302
Mekontso Dessap A, Roche-Campo F, Kouatchet A et al (2012) Natriuretic peptide-driven fluid management during ventilator weaning: a randomized controlled trial. Am J Respir Crit Care Med 186:1256–1263
Cabello B, Thille AW, Roche-Campo F et al (2010) Physiological comparison of three spontaneous breathing trials in difficult-to-wean patients. Intensive Care Med 36:1171–1179
Demoule A, Lefort Y, Lopes M-E, Lemaire F (2004) Successful weaning from mechanical ventilation after coronary angioplasty. Br J Anaesth 93:295–297
Adamopoulos C, Tsagourias M, Arvaniti K et al (2005) Weaning failure from mechanical ventilation due to hypertrophic obstructive cardiomyopathy. Intensive Care Med 31:734–737
Conflicts of interest
Jean-Louis Teboul and Xavier Monnet are members of the Medical Advisory Board of Pulsion Medical Systems. Martin Dres received honoraria for lectures from Pulsion Medical Systems. The other authors have no financial interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: Weaning-induced cardiac dysfunction is one of the most frequent and treatable causes of weaning failure. Detection of the cardiac preload independence by a passive leg raising performed before a spontaneous breathing trial reliably predicts the occurrence of weaning-induced cardiac dysfunction. Whether the PLR test can guide the treatment of weaning failure of cardiac origin should be further investigated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dres, M., Teboul, JL., Anguel, N. et al. Passive leg raising performed before a spontaneous breathing trial predicts weaning-induced cardiac dysfunction. Intensive Care Med 41, 487–494 (2015). https://doi.org/10.1007/s00134-015-3653-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-3653-0