Abstract
Purpose
To investigate the diagnostic performance of Doppler echocardiography (DE) in predicting the outcome of weaning from mechanical ventilation in patients without overt cardiac disease.
Methods
Fifty critical care noncardiac patients who fulfilled predetermined criteria for weaning underwent DE before and at the end of spontaneous breathing trial (pre-SBT/end-SBT, respectively). “Conventional” mitral inflow analysis and “advanced” DE parameters [tissue Doppler imaging (TDI)-derived mitral/tricuspid annular velocities and color M-mode Doppler velocity of propagation (V p)] were used to assess left ventricular (LV) diastolic function/filling pressures. Weaning was considered successful if patients had been extubated after successful SBT and sustained spontaneous breathing for more than 48 h.
Results
Twenty-eight patients (56%) failed weaning: 23 patients failed SBT and 5 required reintubation within 48 h. Weaning failure was associated with the degree of LV diastolic dysfunction at pre-SBT (P = 0.01). Patients who failed weaning presented evidence of increased LV filling pressures at pre-SBT, by demonstrating increased E/E m and E/V p ratios compared with patients with successful outcome (P ≤ 0.004); pre-SBT values of lateral E/E m greater than 7.8 and E/V p greater than 1.51 predicted weaning failure with an area under the curve, sensitivity (%), and specificity (%) of 0.86, 79, and 100, and 0.74, 75, and 73, respectively. Lateral E/E m was the only factor independently associated with weaning failure before SBT; OR (95% CI) 5.62 (1.17–26.96), P = 0.03.
Conclusions
Our findings suggest that LV diastolic dysfunction is significantly associated with weaning outcome in critically ill patients with preserved LV systolic function. An E/E m ratio greater than 7.8 may identify patients at high risk of weaning failure.
Similar content being viewed by others
Introduction
Weaning from mechanical ventilation (MV) is important for critical care patients. Prolonged MV is associated with increased morbidity, cost of care, and mortality in the intensive care unit (ICU) [1]. Several diagnostic tools and treatment algorithms have been suggested in order to predict successful liberation from the ventilator, such as clinical characteristics, respiratory functional indices, and laboratory parameters [1–3]. However, successful weaning still remains a challenging issue.
Hemodynamic alterations and cardiac dysfunction may play a critical role in weaning outcome [4]. After removal of positive-pressure ventilation, negative intrathoracic pressures and sympathetic overstimulation [5] may increase cardiac workload [6] and left ventricular (LV) afterload [7, 8], thus impairing LV compliance; weaning-induced myocardial ischemia [9, 10] or RV dysfunction [11] may also cause LV diastolic dysfunction in weaning. These phenomena, together with increased venous return [7, 8], may increase LV filling pressures and provoke pulmonary edema. Previous investigations pointed out that LV diastolic dysfunction may be a major drawback in weaning [12–14], but study populations included patients with known heart disease and/or concomitant LV systolic dysfunction, who are theoretically at high risk of weaning failure related to pulmonary venous congestion [13, 14]. Yet, the role of LV diastolic dysfunction in the clinical setting of ICU is less clear.
In the present study, we hypothesized that LV diastolic dysfunction may be an important aspect of weaning failure even in ICU patients with preserved LV systolic function without known cardiac pathology. We prospectively evaluated LV diastolic function before and during spontaneous breathing trial (SBT), by performing Doppler echocardiography (DE) in a population who fulfilled predetermined criteria for weaning. In addition, we sought to evaluate the diagnostic performance of DE indices, especially those of LV diastolic function, in predicting weaning outcome.
Patients and methods
In the present prospective study, consecutive sampling was used to recruit patients hospitalized in the General State Hospital of Athens between 2008 and 2009. Patients were included if they had been under MV for more than 72 h and fulfilled predetermined criteria to undergo their first T-tube SBT [1]: (a) clear improvement or resolution of the underlying condition for which the patient had been intubated; (b) alertness, ability to communicate, no further need for sedation; (c) core temperature no greater than 39°C; (d) hemoglobin concentration greater than 7 g/dL; (e) pressure support MV; (f) hemodynamic stability (heart rate no greater than 120/min, systolic blood pressure between 90 and 160 mmHg), no need for vasoactive drugs; (g) ventilatory stability [fraction of inspired oxygen (FiO2) no greater than 0.4, arterial partial pressure of oxygen (PaO2) greater than 60 mmHg, PaO2/FiO2 at least 150, positive end-expiratory pressure no greater than 5 cmH2O]; (h) adequate pulmonary function (respiratory rate no greater than 35/min, tidal volume greater than 5 mL/kg, no significant respiratory acidosis, adequate cough, not excessive tracheobronchial secretions). Exclusion criteria included (a) pre-existing or ensuing (during the period of MV) cardiac pathology (coronary artery disease, severe valvulopathy, cardiomyopathy), (b) atrial fibrillation or other arrhythmias, (c) abnormal LV ejection fraction (less than 0.50) or regional wall motion abnormalities [15], (d) severe neuromuscular disorders. The study was approved by the institutional ethics committee. Next of kin provided written, informed consent for all subjects. Clinical management decisions were taken by the attending physicians whereas all cases were discussed daily in multidisciplinary meeting.
Initially, conventional transthoracic 2D echocardiographic examination was performed to assess global LV/RV function [15]. Patients underwent baseline clinical assessment for evaluation of hemodynamic-respiratory parameters, arterial blood gasses, rapid shallow breathing index (RSBI) [16], and pressure–frequency product (PFP) [17], and DE evaluation followed, while patients were in pressure support ventilation (pre-SBT measurements). MV was discontinued and the patient started breathing spontaneously for 30 min through a T-tube circuit [1]. Follow-up DE (end-SBT) measurements in patients who succeeded in the trial were collected 30 min after SBT initiation; in those who failed the procedure, measurements were obtained before reconnection to the ventilator.
SBT
SBT failure was defined if at least one of the following signs appeared during the trial [1]: (a) objective indices of failure: tachypnea at least 35 breaths/min, use of accessory respiratory muscles, tachycardia at least 140 beats/min, systolic arterial pressure greater than 200 or less than 80 mmHg, hypoxemia—oxygen saturation no greater than 90%, acidosis, arrhythmia; (b) subjective indices: diaphoresis, agitation, depressed mental status, clinical signs of distress. SBT without the presence of the above signs was considered successful and patients were extubated. The decision to initiate or terminate SBT was taken by the attending physician according to the criteria previously detailed. Continuous electrocardiographic monitoring (3-channel recorder using leads I, II, and V2) was used for the detection of ST-segment deviations indicating myocardial ischemia, as suggested [10]; weaning-induced acute cardiac dysfunction–pulmonary edema was evaluated as previously suggested [13, 18]. Noninvasive ventilation (NIV) was not used as per protocol to assist patients and to avoid reintubation. Attending physicians were not aware of DE findings.
Weaning outcomes
Weaning success was defined as the ability of patients to pass the SBT and to remain on spontaneous ventilation more than 48 h from extubation [1].
Doppler echocardiographic study
Doppler echocardiography was performed from the apical 4-chamber view with an ultrasound device (Philips, XD11 XE, Andover, MA, USA), equipped with the tissue Doppler imaging program and a phased array multifrequency transducer. Doppler signals were recorded along with the electrocardiogram and respiratory cycle waveform at a sweep speed of 100 mm/s and were stored digitally in the hardware for later analysis. Off-line analysis was carried out by an experienced cardiologist blinded to patients’ identity and clinical status. The average of three end-expiratory cycles was used [19] (see also online data supplement).
Mitral inflow pulsed-wave Doppler signals [peak velocities of early (E) and late (A) LV diastolic filling, E-wave deceleration time (DTE)], tissue Doppler imaging (TDI)-derived peak systolic (S m)/early diastolic (E m)/late diastolic (A m) velocities at the lateral/septal mitral annulus and lateral tricuspid annulus, and color M-mode Doppler velocity of propagation (V p) were obtained from the apical 4-chamber view, as suggested [19]. Analysis was also performed for “conventional” (E/A ratio) and “advanced” (E/E m, E/V p) DE indices of LV filling pressures [19].
LV diastolic function was graded by means of DE, as suggested [19]. Accordingly, our patients were classified as having (1) normal diastolic function, (2) grade I (mild) diastolic dysfunction, or (3) grade II–III (moderate to severe) diastolic dysfunction.
Duration of ultrasound studies was 3 ± 0.3 min. The interobserver reproducibility of Doppler and TDI parameters was evaluated by two observers in 20 randomly selected patients (Table 1). Mean (±standard error, SE)% absolute difference between evaluation of DE parameters was overall 2.8 ± 0.3.
Data analysis
Descriptive statistics were used to summarize the baseline characteristics and the results were expressed as mean ± SE. The t test or χ2 (or Fisher’s test) was applied for comparisons between groups. Correlations between variables were assessed appropriately either by Pearson’s r 2 or by Spearman’s rho. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of clinical/Doppler parameters in identifying patients who failed SBT and/or weaning. The area under the curve (AUC) and the respective 95% confidence interval (95% CI) was estimated according to Hanley and McNeil [20]. The AUC curves were compared using the method described by DeLong et al. [21]. Multiple logistic regression analysis was used to examine the effect of multiple clinical/echocardiographic risk factors in weaning failure. A P value less than 0.05 was considered statistically significant. The statistical package SPSS 13.0 (Chicago, IL, USA) was used. ROC analyses were performed using subroutines in Compaq Visual Fortran 90 with the IMSL library (Edition 6.6.0, 000. Polyhedron Software Ltd., Witney, UK).
Results
Fifty patients participated in the study (Table 2). Twenty-six patients had severe neuro-critical illness on admission [traumatic brain injury (n = 17), intracranial hemorrhage (n = 4), scheduled neurosurgery (n = 5)]. Twenty-one patients had a history of chronic obstructive pulmonary disease (COPD). Prehospital data for GOLD classification [22] were available for 12 COPD patients: stage II (n = 9); stage III–IV (n = 3).
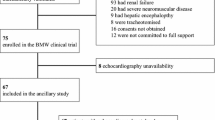
Overall, 28 (56%) out of 50 patients failed weaning (Fig. 1): 23 (46%) failed SBT and 5 passed SBT but required reintubation within 48 h. Fifteen out of 23 patients who failed SBT (65%) presented clinical signs suggestive of pulmonary edema (PE) [13, 18], such as rales, wheezes, bronchospasm, diaphoresis, adrenergic overstimulation; four of them exhibited reversible ischemic ST-segment deviations on SBT. Four patients failed SBT primarily due to hypoxemia-respiratory acidosis and four others due to consciousness level disorders. Reasons for reintubation within 48 h were respiratory failure (n = 3; two of them presented signs compatible with PE), decreased consciousness–inability to protect airway (n = 2). NIV was used only in three out of five patients who were reintubated within 48 h after initially successful SBT. Forty-three patients (86%) required inotropes/vasopressors upon admission; none needed such agents during the 48-h weaning period. None of our patients was pretreated with diuretics 8 h before SBT; four patients received furosemide in the 48 h preceding the trial.
Eleven of the 28 patients were extubated 11 ± 3.3 days after SBT; eight patients were discharged from ICU with tracheostomy. All 22 patients who succeeded weaning were discharged from ICU. Overall, ICU mortality was 18% (9/50).
LV function assessed by Doppler echocardiography
Baseline DE-estimated diastolic function is shown in Fig. 2. Twenty-three (46%) patients presented normal function, seven (14%) demonstrated mild (grade I) and 20 (40%) moderate to severe (grade II–III) diastolic dysfunction. Patients with diastolic dysfunction were older (P < 0.001), with a history of COPD (P < 0.001), arterial hypertension (P = 0.021), and presented thicker LV walls (P < 0.001), increased LV mass index (P < 0.001) and relative wall thickness (RWT) (P < 0.001); they also presented decreased mitral annular velocities E m (P < 0.001) and S m (P ≤ 0.003), and increased E/E m ratios (P ≤ 0.001) at pre-SBT. Overall, LV diastolic function tended to deteriorate between pre-SBT and end-SBT (P = 0.051). Six patients (12%) presented significant LV diastolic deterioration moving towards higher LV diastolic function grades; overall, no patient improved LV function grade. Fluid balance (mean of 3 days preceding SBT) was associated neither with the degree of diastolic function at pre-SBT (ANOVA, P = 0.45), nor with SBT-induced changes in lateral E/E m (P = 0.68). COPD patients presented higher pre-SBT E/E m values (P ≤ 0.003) and greater SBT-induced increases in averaged E/E m (P = 0.05).
Relationship between clinical and echocardiographic parameters and weaning
Baseline clinical and echocardiographic characteristics relating to weaning outcomes are shown in Table 3. Αcute changes (Δ%) in DE markers reflecting ventricular function and LV filling pressure alterations during SBT did not differ between patients who succeeded in and patients who failed the weaning trial (see online data supplement for details).
Diagnostic performance of clinical and echocardiographic parameters
The diagnostic performance and threshold values of various clinical and cardiac Doppler indices at pre-SBT in predicting weaning outcome are shown in Table 4. Pre-SBT lateral E/E m greater than 7.8 demonstrated the greatest performance in predicting weaning failure among various variables; pre-SBT lateral E/E m AUC was 0.86, specificity 100%, and positive predictive value 100%. Notably, pre-SBT E/E m greater than 7.8 and E/V p greater than 1.6 could accurately identify weaning failure and need for reintubation in the subgroup of our patients (n = 27) who had successful SBT initially (sensitivity 100 and 80% and specificity 100 and 82%, for E/E m and E/V p, respectively). ROC curves for pre-SBT values of lateral E/E m ratio, E/V p ratio, RSBI, and PFP in predicting weaning failure are presented in Fig. 3; E/E m differed significantly from the other three markers. Multivariate analysis showed that best predictors for weaning failure were E/E m OR (95% CI) 5.62 (1.17–26.96), P = 0.03 and LV mass index 1.13 (0.98–1.3), P = 0.09 (see online data supplement for comparison among ROC curves and for multivariate analysis).
ROC curves for two echocardiographic pre-SBT markers of left ventricular filling pressures (E/E m, E/V p) and two clinical parameters (RSBI and PFP) in predicting weaning failure. E/E m ratio of transmitral inflow E-wave velocity to TDI-derived early diastolic velocity at the lateral border of the mitral annulus; E/V p transmitral E-wave velocity to color M-mode Doppler velocity of propagation ratio, RSBI rapid shallow breathing index, PFP pressure–frequency product, AUC area under the curve
Discussion
The present study suggests that LV diastolic dysfunction and increased LV filling pressures may be involved in the pathophysiology of weaning failure in ICU patients with preserved systolic function. Patients who failed weaning presented significantly worse diastolic function and increased E/E m or E/V p ratios before SBT, compared with those with successful weaning. Furthermore, our findings suggest that DE indices may predict the outcome of weaning from mechanical ventilation in ICU patients with preserved LV systolic function. Thus, E/E m ratios at pre-SBT presented the greatest diagnostic performance in predicting weaning failure (AUC 0.86) among several established weaning markers, such as the RSBI [3, 16] or pressure–frequency product [17]. In this respect, performing DE before the initiation of an SBT may be of value in weaning-related decisions by assisting clinical data.
Diastolic dysfunction is frequent in ICU patients [23–26]. However, data assessing the role of diastolic dysfunction in weaning from MV are sparse [12–14]. We hypothesized that diastolic dysfunction might be a cause of unsuccessful weaning in ICU patients with preserved LV systolic function. We found that patients with moderate to severe LV diastolic dysfunction at pre-SBT exhibited significantly increased weaning failure rates (Fig. 2). We also noted that during SBT, LV diastolic function tended to deteriorate. A plausible explanation for the relationship between diastolic dysfunction and weaning failure might be that patients with diastolic dysfunction present diastolic heart failure and pulmonary edema on spontaneous ventilation. Transition from positive-pressure ventilation to spontaneous breathing induces negative intrathoracic pressures with increased venous return and sympathetic overstimulation [5] that may increase LV preload–afterload [7, 8] and cardiac workload [6], thus impairing LV compliance; weaning-induced myocardial ischemia [9, 10] or RV dysfunction [11], may also deteriorate pre-existing LV diastolic dysfunction and may increase LV filling pressures and thus provoke pulmonary edema. Notably, it was recently reported that patients with diastolic dysfunction may present impaired diastolic reserve under stressful conditions [27], and this possibly explains weaning intolerance. In line with the above, other authors suggested that weaning outcomes may improve with the application of strategies aiming to manage heart failure (i.e., diuretics, nitroglycerin) [17, 28, 29].
In our cohort, we found that patients who failed weaning presented known precipitating factors for LV diastolic dysfunction (i.e., age, arterial hypertension, increased wall thickness and LV mass, and COPD) [30]. Furthermore, we found that they presented DE indices (increased E/E m and E/V p) indicating significantly elevated LV filling pressures, and decreased S m and E m mitral annular velocities compared with patients with successful weaning. Although decreased S m is usually found in patients with systolic dysfunction [31], depressed S m velocities are also found in patients with normal systolic function who present diastolic heart failure [32]. Given the association between depressed S m and E m values and ischemia [33], as well as the fact that rate–pressure product (heart rate × systolic blood pressure), a global index of myocardial workload and oxygen demand [6], increased more during failing trials (data shown in online data supplement—Table E4), our findings suggest that myocardial ischemia might also be implicated in weaning failure. However, ST-segment dynamic deviations occurred in only four patients in our study. In this respect, a hypothesis that myocardial ischemia is an explanation for these phenomena might be challenged and this has to be taken into consideration in the interpretation of our results.
Another potential mechanism of impaired LV diastolic function in weaning, especially in COPD patients, is that increases in RV afterload because of hypoxemia and pulmonary dynamic hyperinflation, together with a simultaneous increase in venous return, may cause RV dysfunction. The latter may impede LV diastolic filling through an interventricular interdependence mechanism [11]. RV dysfunction might have been implicated in the weaning of our COPD subpopulation, whose DE markers of LV filling pressures were indicative of significantly elevated values at baseline and greater changes during SBT compared with patients without COPD. Positive fluid balance that could have contributed to volume overload and increased LV filling pressures was not associated with the degree of diastolic function at pre-SBT or with SBT/weaning outcomes in our study. Thus, one could assume that LV diastolic dysfunction may play a more important role than volume state in SBT-induced increases of LV filling pressures.
In the present study, “advanced” DE markers of LV filling pressures (E/E m and E/V p) at pre-SBT presented considerable diagnostic performance in predicting weaning failure; E/E m greater than 7.8 at the lateral border of mitral annulus and E/V p greater than 1.51 before the initiation of SBT predicted unsuccessful weaning with sensitivity (%) and specificity(%) of 79/75 and 100/73, respectively (Table 4). These indices retained their predictive value even in the subset of patients who initially passed SBT; therefore, E/E m and E/V p at pre-SBT could identify patients who will probably fail to remain in spontaneous breathing regardless of SBT result. However, SBT-induced acute alterations in E/E m and E/V p, although tending to reach statistical significance, were not predictive of weaning outcomes (online data supplement—Tables E1, E4). A plausible explanation might be that myocardial viscoelastic properties at baseline are impaired in patients who failed weaning; LV filling pressures, as indicated by increased E/E m and E/V p, may be higher in these patients at baseline, close to a critical point beyond which pulmonary edema develops. Our results support previous studies [13, 17] which reported that the echocardiographic (or by cardiac biomarkers) assessment of LV function before the initiation of weaning trial may be of greater importance than monitoring the acute changes that occur during SBT.
Interestingly, other investigators have reported similar E/E m values as predictors of invasively estimated increased LV filling pressures, either in MV [34] or during SBT [12], whereas another study [13] with similar design to ours showed that E/E m was significantly higher in patients who could not be weaned from the ventilator compared with those who did. However, in the latter study, patients with increased E/E m ratios also presented significantly decreased LV ejection fractions; our findings point out that increased E/E m ratio can also be found in patients without overt cardiac disease.
Our findings are based on the study of DE indices and one could argue that the diagnostic performance of E/E m and E/V p as reliable estimates of LV filling pressures and the feasibility of accurate acquisition of E/V p have been challenged [19]. We certainly acknowledge this skepticism. However, we used lateral E/E m ratios, which enable the prediction of increased LV filling pressures in patients with LVEF greater than 50% [35], even in the clinical settings of mechanical ventilation [36] or diastolic heart failure [37]. On the other hand, E/V p ratios may be helpful in the evaluation of LV diastolic function in patients with normal systolic function [35, 38] even during acute changes in cardiac loading conditions [39]. Yet, reproducible V p measurements can be obtained by following published recommendations [19].
Our investigation provided evidence suggesting that LV diastolic dysfunction may be implicated in the pathophysiologic mechanisms related to unsuccessful weaning in our cohort. However, it should be underlined here that it is not a “scoop” that patients with normal LV diastolic function succeed in weaning, as almost half of our patients in the grade I group also failed; unfortunately, there were only seven patients in this subgroup and other markers that might have intensified our findings, such as brain natriuretic peptide (BNP) plasma levels [17, 18], were not assessed, and these are limitations of our study. It should also be noted that our results did not derive from the wider ICU population requiring weaning from MV, but from an inhomogeneous population with many COPD and neuro-critical patients (overall 84%) ventilated for more than 72 h; in such populations weaning failure rates are expected to be high [2].
In conclusion, our findings suggest that in patients with no overt cardiac disease and with preserved LV systolic function, LV diastolic dysfunction may play an important role in weaning failure. DE indices, particularly E/E m at the lateral border of the mitral annulus, may be helpful to identify patients at high risk of weaning failure. We acknowledge that further evidence is required before recommending echocardiography as a standard tool during weaning in the everyday clinical setting in the ICU. However, our data provide evidence suggesting that DE indices may assist in weaning from MV and may guide weaning-related therapeutic interventions (i.e., volume contraction, nitrate infusion) in the future.
References
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T (2007) Weaning from mechanical ventilation. Eur Respir J 29:1033–1056
Vallverdú I, Calaf N, Subirana M, Net A, Benito S, Mancebo J (1998) Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med 158:1855–1862
Yang KL, Tobin MJ (1991) A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 324:1445–1450
Lamia B, Monnet X, Teboul JL (2005) Weaning-induced cardiac dysfunction. In: Yearbook of intensive care and emergency medicine. 1st edn. Springer, Heidelberg, pp 239–245
Kennedy SK, Weintraub RM, Skillman JJ (1977) Cardiorespiratory and sympathoadrenal responses during weaning from controlled ventilation. Surgery 82:233–240
Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y (1978) The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 57:549–556
McGregor M (1979) Current concepts: pulsus paradoxus. N Engl J Med 301:480-482
Buda AJ, Pinsky MR, Ingels NB Jr, Daughters GT 2nd, Stinson EB, Alderman EL (1979) Effect of intrathoracic pressure on left ventricular performance. N Engl J Med 301:453–459
Hurford WE, Favorito F (1995) Association of myocardial ischemia with failure to wean from mechanical ventilation. Crit Care Med 23:1475–1480
Frazier SK, Brom H, Widener J, Pender L, Stone KS, Moser DK (2006) Prevalence of myocardial ischemia during mechanical ventilation and weaning and its effects on weaning success. Heart Lung 35:363–373
Boussuges A, Pinet C, Molenat F, Burnet H, Ambrosi P, Badier M, Sainty JM, Orehek J (2000) Left atrial and ventricular filling in chronic obstructive pulmonary disease. An echocardiographic and Doppler study. Am J Respir Crit Care Med 162:670–675
Lamia B, Maizel J, Ochagavia A, Chemla D, Osman D, Richard C, Teboul JL (2009) Echocardiographic diagnosis of pulmonary artery occlusion pressure elevation during weaning from mechanical ventilation. Crit Care Med 37:1696–1701
Caille V, Amiel JB, Charron C, Belliard G, Vieillard-Baron A, Vignon P (2010) Echocardiography: a help in the weaning process. Crit Care 14:R120
Bernard F, Denault A, Babin D, Goyer C, Couture P, Couturier A, Buithieu J (2001) Diastolic dysfunction is predictive of difficult weaning from cardiopulmonary bypass. Anesth Analg 92:291–298
Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I et al (1989) Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2:358–367
El-Khatib MF, Jamaleddine GW, Khoury AR, Obeid MY (2002) Effect of continuous positive airway pressure on the rapid shallow breathing index in patients following cardiac surgery. Chest 121:475–479
Mekontso-Dessap A, de Prost N, Girou E, Braconnier F, Lemaire F, Brun-Buisson C, Brochard L (2006) B-type natriuretic peptide and weaning from mechanical ventilation. Intensive Care Med 32:1529–1536
Grasso S, Leone A, De Michele M, Anaclerio R, Cafarelli A, Ancona, Stripoli T, Bruno F, Pugliese P, Dambrosio M, Dalfino L, Di Serio F, Fiore T (2007) Use of N-terminal pro-brain natriuretic peptide to detect acute cardiac dysfunction during weaning failure in difficult-to-wean patients with chronic obstructive pulmonary disease. Crit Care Med 35:96–105
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10:165–193
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J (2007) Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176:532–555
Sturgess DJ, Marwick TH, Joyce CJ, Jones M, Venkatesh B (2007) Tissue Doppler in critical illness: a retrospective cohort study. Crit Care 11:R97
Jafri SM, Lavine S, Field BE, Bahorozian MT, Carlson RW (1990) Left ventricular diastolic function in sepsis. Crit Care Med 18:709–714
Wu CK, Lee JK, Chiang FT, Yang CH, Huang SW, Hwang JJ, Lin JL, Tseng CD, Chen JJ, Tsai CT (2011) Plasma levels of tumor necrosis factor-α and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit Care Med 39:984–992
Miyamoto M, McClure DE, Schertel ER, Andrews PJ, Jones GA, Pratt JW, Ross P, Myerowitz PD (1998) Effects of hypoproteinemia-induced myocardial edema on left ventricular function. Am J Physiol 274:H937–H944
Chattopadhyay S, Alamgir MF, Nikitin NP, Rigby AS, Clark AL, Cleland JG (2010) Lack of diastolic reserve in patients with heart failure and normal ejection fraction. Circ Heart Fail 3:35–43
Lemaire F, Teboul JL, Cinotti L, Giotto G, Abrouk F, Steg G, Macquin-Mavier I, Zapol WM (1988) Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology 69:171–179
Routsi C, Stanopoulos I, Zakynthinos E, Politis P, Papas V, Zervakis D, Zakynthinos S (2010) Nitroglycerin can facilitate weaning of difficult-to-wean chronic obstructive pulmonary disease patients: a prospective interventional non-randomized study. Crit Care 14:R204
European Study Group on Diastolic Heart Failure (1998) How to diagnose diastolic heart failure. Eur Heart J 19:990–1003
Yuda S, Inaba Y, Fujii S, Kokubu N, Yoshioka T, Sakurai S, Nishizato K, Fujii N, Hashimoto A, Uno K, Nakata T, Tsuchihashi K, Miura T, Ura N, Natori H, Shimamoto K (2006) Assessment of left ventricular ejection fraction using long-axis systolic function is independent of image quality: a study of tissue Doppler imaging and m-mode echocardiography. Echocardiography 23:846–852
Vinereanu D, Nicolaides E, Tweddel AC, Fraser AG (2005) “Pure” diastolic dysfunction is associated with long-axis systolic dysfunction. Implications for the diagnosis and classification of heart failure. Eur J Heart Fail 7:820–828
García-Fernández MA, Azevedo J, Moreno M, Bermejo J, Pérez-Castellano N, Puerta P, Desco M, Antoranz C, Serrano JA, García E, Delcán JL (1999) Regional diastolic function in ischaemic heart disease using pulsed wave Doppler tissue imaging. Eur Heart J 20:496–505
Vignon P, AitHssain A, François B, Preux PM, Pichon N, Clavel M, Frat JP, Gastinne H (2008) Echocardiographic assessment of pulmonary artery occlusion pressure in ventilated patients: a transoesophageal study. Crit Care 12:R18
Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF (2003) Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol 91:780–784
Combes A, Arnoult F, Trouillet JL (2004) Tissue Doppler imaging estimation of pulmonary artery occlusion pressure in ICU patients. Intensive Care Med 30:75–81
Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschöpe C (2007) Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 116:637–647
González-Vilchez F, Ayuela J, Ares M, Mata NS, González AG, Durán RM (2002) Comparison of Doppler echocardiography, color M-mode Doppler, and Doppler tissue imaging for the estimation of pulmonary capillary wedge pressure. J Am Soc Echocardiogr 15:1245–1250
Licker M, Cikirikcioglu M, Inan C, Cartier V, Kalangos A, Theologou T, Cassina T, Diaper J (2010) Preoperative diastolic function predicts the onset of left ventricular dysfunction following aortic valve replacement in high-risk patients with aortic stenosis. Crit Care 14:R101
Acknowledgments
J. P. drafted the manuscript and participated in the collection of data; D. M. drafted part of the manuscript and revised the manuscript for important intellectual content; T. S., D. K., and A. K. participated in the collection of data; El. Z. performed statistical analysis; E. Z. performed off-line echocardiographic analysis; E. Z. and G. K. contributed equally in the study design and motivated the study.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Papanikolaou, J., Makris, D., Saranteas, T. et al. New insights into weaning from mechanical ventilation: left ventricular diastolic dysfunction is a key player. Intensive Care Med 37, 1976–1985 (2011). https://doi.org/10.1007/s00134-011-2368-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2368-0