Abstract
Purpose
To compare cardiovascular and respiratory responses to different spontaneous breathing trials (SBT) in difficult-to-wean patients using T-piece and pressure support ventilation (PSV) with or without positive end-expiratory pressure (PEEP).
Methods
Prospective physiological study. Fourteen patients who were monitored with a Swan-Ganz catheter and had failed a previous T-piece trial were studied. Three SBTs were performed in random order in all patients: PSV with PEEP (PSV-PEEP), PSV without PEEP (PSV-ZEEP), and T-piece. PSV level was 7 cmH2O, and PEEP was 5 cmH2O. Inspiratory muscle effort was calculated, and hemodynamic parameters were measured using standard methods.
Results [median (and interquartile range)]
Most patients succeeded in the PSV-PEEP (11/14) and PSV-ZEEP (8/14) trials, but all failed the T-piece trial. Patient effort was significantly higher during T-piece than during PSV with or without PEEP [esophageal pressure-time product was 292 (238–512), 128 (58–299), and 148 (100–465) cmH2O·s/min, respectively, p < 0.05]. Left ventricular heart failure was observed in 11 of the 14 patients during the T-piece trial. Pulmonary artery occlusion pressure and respiratory rate were significantly higher during T-piece than with PSV-PEEP [21 (18–24) mmHg versus 17 (14–22) mmHg, p < 0.05 and 27 (21–35) breaths/min versus 19 (16–29) breaths/min, p < 0.05 respectively]. Tidal volume was significantly lower during the T-piece trial.
Conclusion
In this selected population of difficult-to-wean patients, PSV and PSV plus PEEP markedly modified the breathing pattern, inspiratory muscle effort, and cardiovascular response as compared to the T-piece. Caregivers should be aware of these differences in SBT as they may play an important role in weaning decision-making.
Similar content being viewed by others
Introduction
Early identification of patients able to progress to spontaneous breathing promotes shorter duration of mechanical ventilation and reduces complications [1]. Once a physician deems that a patient is ready to breathe without ventilatory assistance, a screening test is performed. If this is successful, a spontaneous breathing trial (SBT) of variable duration (usually from 30 to 120 min) is conducted. The SBT is needed to confirm the patient’s ability to breathe without assistance [2]. Switching from mechanical to spontaneous ventilation can decrease left ventricular performance by increasing preload and afterload [3], and unmask latent left ventricular heart failure (LVHF) [4]. In difficult-to-wean patients, T-piece spontaneous breathing often induces an increase in left ventricular filling pressure [5]. Early detection of cardiac origin as a cause of respiratory distress during a weaning trial is relevant since treatment with vasodilators and/or diuretics will usually improve clinical symptomatology and may hasten extubation [4].
The SBT is generally performed by disconnecting the patient from the ventilator and attaching a T-piece to the endotracheal tube. Some authors, however, prefer to use low levels of pressure support ventilation (PSV) with or without positive end-expiratory pressure (PEEP) [6, 7]. It has been reported that the rate of failure may be higher with a T-piece trial than with a PSV trial [8, 9], possibly because respiratory muscle energy expenditure is greater with the former [10–12]. A less demanding trial in terms of respiratory muscle effort, especially when PEEP is added to PSV, may also prevent the development of LVHF and thus blunt the clinical signs and symptoms of intolerance to unassisted T-piece spontaneous breathing.
In difficult-to-wean patients (i.e., those who had failed a first confirmatory T-piece trial), SBTs using a T-piece or low levels of PSV with and without PEEP have not been systematically compared in a physiological study exploring both cardiovascular and respiratory responses. We hypothesized that these three trials (T-piece, PSV with PEEP, and PSV without PEEP) elicit different responses of the cardiovascular and respiratory systems, especially in at risk populations of difficult-to-wean patients. Some of the results of this study have been previously reported in abstract form [13].
Patients and methods
This study was approved by the Ethics Committee of Sant Pau Hospital. All patients and/or their surrogates were informed, and written consent was given prior to inclusion in the study.
The physiological study was performed over a 10-month period in a 16-bed medical-surgical intensive care unit of a university hospital. All consecutive patients requiring mechanical ventilation were prospectively screened and followed until they fulfilled usual weaning criteria.
Our at-risk population consisted of those patients who failed a confirmatory SBT with a T-piece within 60 min of its initiation and already had a Swan-Ganz catheter in place for clinical reasons. After failing the first SBT with a T-piece, patients were reconnected to the ventilator in volume assist-control mode (ACV), and a double balloon esophageal-gastric catheter (see physiological measurements, below) was passed through a nostril after topical anesthesia. After clinical stabilization under ACV (baseline), three SBTs were performed in random order in all participants: T-piece, PSV-PEEP (PSV level of 7 cmH2O with a PEEP of 5 cmH2O), and PSV-ZEEP (PSV level of 7 cmH2O and zero PEEP). A PSV level of 7 cmH2O was set since it has been used in other studies and has been found to compensate for the impedance of the ventilator and its circuits [8]. Heated humidifiers were used during PSV to avoid an increase in work of breathing induced by the deadspace and resistance of the heat and moisture exchangers [14]. The SBTs were stopped if any signs of poor tolerance developed and were considered a success if no signs of poor tolerance occurred within 60 min of initiation. Between each test, the patient was ventilated in ACV for 20–30 min to achieve a cardio-respiratory status equal to baseline.
At the time of the study, a Doppler-echocardiography machine was not available in our department. Consequently, our patients were frequently monitored using a Swan-Ganz catheter.
Spontaneous breathing trial failure
The initial SBT with a T-piece was performed in all patients who fulfilled the usual criteria [15]: SpO2 ≥ 90% with a FiO2 ≤ 0.5 and a PEEP ≤ 5 cmH2O, hemodynamic stability defined by absence of vasopressors, no continuous sedation, Glasgow Coma Scale ≥ 10 (maximum score is 15 points), and a respiratory rate to tidal volume quotient <100 breaths/min/l.
The SBT with a T-piece was considered a failure if the patient developed one of the following signs or symptoms of poor tolerance within 60 min of its initiation: respiratory rate greater than 35 breaths/min, increased accessory muscle activity, unbearable dyspnea, persistent decrease in SpO2 below 90%, heart rate above 140 beats/min or increased by 20%, arrhythmias, drop in systolic blood pressure below 80 mmHg or a rise above 160 mmHg, agitation, diaphoresis, or depressed mental status. The same criteria were used to define SBT failure when PSV-PEEP and PSV-ZEEP were tested.
Physiological measurements
Pulmonary artery occlusion pressure (PAOP) was recorded at the end of expiration. Measurements were performed at the end of each SBT, and LVHF was defined by a PAOP ≥18 mmHg [16]. PAOP was measured after systematic verification of zero reference at the level of right atrium.
Cardiac output was measured by the thermodilution method using 10 ml of saline at room temperature, and the average of three measurements was taken. Pulmonary artery blood was withdrawn in commercially available pre-heparinized plastic syringes and was immediately analyzed with an ABL 520 gas analyzer (Radiometer, Copenhaguen). Airflow and the airway, esophageal, and gastric pressures were measured with standard methodology and proper testing [17]. Signals were digitized and stored for subsequent calculations. Details regarding these measurements and signal acquisition are provided in the Electronic Supplementary Material (ESM).
Physiological assessment of patient effort
Breathing pattern and minute ventilation were determined from the airflow signal. Respiratory muscle energy expenditure was quantified using the esophageal pressure-time product (PTP), taking intrinsic positive end-expiratory pressure (PEEPi) into account [10, 18]. The inspiratory work of breathing (WOB) per breath was computed according to common methods [18–20]. Details regarding these calculations are provided in the ESM.
Statistical analysis
Continuous variables are expressed as median and interquartile range (IQR). We compared the values obtained at the end of the three SBTs (T-piece, PSV-PEEP, and PSV-ZEEP), before reconnecting the patient to ACV. To test differences between SBTs, we used the nonparametric Friedman test because of the small sample size. If a significant difference appeared, a Wilcoxon test was used to perform pairwise comparisons. Statistical analysis was done using the SPSS statistical software (SPSS 13, Chicago, IL).
Results
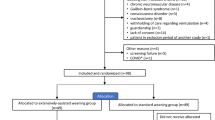
The physiological study comparing the three SBT was performed in 14 patients. Twenty-nine patients could not be included. The study flowchart is shown in Fig. 1. Patients’ clinical characteristics are given in Table 1.
Ventilatory parameters, gas exchange, and inspiratory muscle effort
Although there were no significant differences between ACV and PSV-PEEP in terms of respiratory rate and tidal volume, there was a marked difference between PSV trials and T-piece trial (Table 2). PEEPi increased significantly during PSV-ZEEP and T-piece (Table 2). The FiO2 did not differ: it was 0.38 (0.3–0.44) during all the study. Arterial blood gases showed a significant increase in PaCO2 and a significant decrease in pH during T-piece, whereas PaO2 did not significantly change (Table 2).
Patients’ inspiratory effort increased markedly from both ACV and PSV-PEEP to PSV-ZEEP and T-piece, either expressed as PTP or WOB. Data are shown in Table 2, and representative tracings can be seen in Fig. 2.
Major expiratory muscle activity with positive pressure swings higher than 5 cmH2O was observed in only three patients during PSV-ZEEP and T-tube trial and in no patients during PSV-PEEP.
Hemodynamic parameters and SBT tolerance
Four patients had a PAOP ≥18 mmHg while ventilated in ACV (nos. 1, 3, 8, and 12). PAOP was 18 mmHg or higher in 7 patients during PSV-PEEP, in 9 patients during PSV-ZEEP, and in 11 patients during the T-piece trial. Nearly 80% (8/11) of patients who had a PAOP ≥18 mmHg during the T-piece trial failure had prior cardiovascular disease: five had systemic arterial hypertension, and three had myocardial ischemia (Table 1). In addition, more than 50% (6/11) also had a chronic obstructive pulmonary disease (Table 1). Although PAOP showed a trend to increase from ACV to PSV-PEEP [15 (12–18) mmHg and 17 (14–22), p = 0.05], it increased markedly during PSV-ZEEP [20 (15–25) mmHg] and during the T-piece trial [21 (18–24) mmHg]. Cardiac output and the mixed venous oxygen saturation remained unchanged (Table 3). Systolic blood pressure and heart rate increased significantly during T-piece (Table 3).
All patients failed the T-piece trial within the first 60 min of disconnection, but 11 (79%) successfully completed the PSV-PEEP trial, and 8 (57%) successfully completed the PSV-ZEEP trial. Individual data are shown in Table 4.
A cumulative (from the moment of admission) positive fluid balance was observed in four patients on the study day, but in no patients on the extubation day. Extubation was performed, on average, 5 days after the study day. The cumulative fluid balance was significantly lower when extubation was carried out: −935 (−3,343 to 342) ml the study day versus −5,065 (−11,710 to −3,530) ml the extubation day, p < 0.01 (Table 4).
Discussion
This study suggests that in our selected difficult-to-wean patients, clinical and physiological responses differ depending on the type of SBT used to ascertain whether or not a patient is ready for extubation. The breathing pattern and cardiovascular responses differed considerably among the three SBTs. Eleven of 14 (79%) patients who failed a T-piece trial successfully completed a PSV-PEEP trial, and 8/14 (57%) succeeded the PSV-ZEEP trial. PAOP that was normal during ACV increased to over 18 mmHg in 7/10 patients during T-piece, in 5/10 during PSV-ZEEP, and in only 3/10 during PSV-PEEP. This suggests that both mechanical ventilation and PEEP protected left ventricular function. In these selected individuals, the use of even low levels of ventilatory support may confuse clinicians about the clinical tolerance to unassisted spontaneous breathing.
T-piece versus pressure support trials: clinical implications
We found that the patient effort required during a T-piece trial was higher than during a PSV trial. Furthermore, we found that the effort was higher during the PSV-ZEEP trial than during the PSV-PEEP trial. As long as support was added, the failure rate during SBT decreased. Esteban et al. [8] found that more patients failed the T-piece trial (22%) than the PSV trial (14%). Ezingeard et al. [9] performed a PSV-ZEEP trial in 31 patients immediately after a T-piece trial failure and found that the PSV trial was successful in most patients (68%), a similar figure to our 57%. In these two studies [8, 9], patients who succeeded a PSV trial were extubated without a significant increase in re-intubation rate as compared to T-piece. Thus, it could be argued that we delayed extubation of those individuals who succeeded a PSV trial. However, we studied a selected population of difficult-to-wean patients: the median duration of mechanical ventilation was 16 days, they had failed a previous SBT with a T-piece, they also had multiple comorbidities, and they were relatively older than the populations in the other studies. In the study by Esteban et al. [8], two-thirds of the patients were successfully extubated after 6 days of mechanical ventilation and a first weaning trial.
In the study by Ezingeard et al. [9], no patient had cardiac failure, and patients were successfully extubated after 8–9 days of mechanical ventilation. In addition, their study was conducted in patients who had been intubated with relatively narrow endotracheal tubes (7.5-mm internal diameter), and the vast majority of patients who were considered as T-piece trial failure were deemed so only on the basis of an increased respiratory rate. It is well known that even low levels of PSV profoundly modify the breathing pattern [21–23] and that narrow endotracheal tubes may promote an increase in respiratory rate [24].
Effects of PEEP and role of LVHF
We found that more patients succeeded the PS trial when a PEEP of 5 cmH2O was added. There are several possible explanations. First, the application of PEEP was associated with a marked decrease in respiratory muscle energy expenditure. This is in agreement with previous reports that found that the magnitude of patient effort decreased by 30–40% [10]. Second, PEEP attenuated PEEPi, and according to findings by other authors [19, 25], this could also have reduced the WOB required to trigger the ventilator. Thirdly, PAOP values were decreased when PEEP was added. Indeed, PEEP is known to improve left ventricular performance [26], especially in patients with LVHF [27].
The large swings in pleural pressure that occurred when PEEP was not used (i.e., T-piece and PSV-ZEEP) increased afterload, thereby decreasing left ventricular performance [3]. The increase in PEEPi that was observed when external PEEP was withdrawn could also increase right ventricular afterload [5]. These phenomena may generate a vicious circle by further increasing both inspiratory effort and myocardial oxygen demand; this could potentially cause myocardial ischemia, mitral regurgitation, and a magnification in pulmonary edema, specially in patients with abnormal systolic ventricular function [28]. Interestingly, Jubran et al. [29] found a marked increase in the WOB due to an increase in inspiratory airway resistance and the development of PEEPi in patients who failed a T-piece trial. These abnormalities are compatible with an early phase of a cardiogenic pulmonary edema [29].
In a subsequent study, Jubran et al. [30] found a marked increase in PAOP (from 11 to 27 mmHg) and a drop in mixed venous oxygen saturation in patients who failed a T-piece trial. These patients were unable to increase their cardiac output to counteract the increase in oxygen consumption during spontaneous ventilation. In contrast, we did not find a drop in mixed venous oxygen saturation during failed SBTs. The discrepancies between the two studies could have several explanations. Our patients had a well-preserved cardiac output, suggesting that systolic function was relatively normal. Although we did not perform an echocardiography exam, we speculate that the main cause of LVHF might be diastolic dysfunction. Eight of our patients had cardiovascular comorbidities (hypertension or myocardial infarction) and had a median age of 69 years. It has been described that populations like this are prone to develop diastolic dysfunction [31–33].
The baseline PAOP was relatively high in our study, probably due to hypervolemia. Overload associated with a normal or high cardiac output may also avoid insufficient oxygen transport, and our patients were rapidly reconnected to the ventilator when the SBT failure occurred, thus minimizing the likelihood of a drop in mixed venous oxygen saturation. Finally, we cannot rule out that myocardial ischemia and/or dynamic mitral regurgitation could have contributed to the development of an increased PAOP [5, 28, 32, 34].
Limitations
As in other studies in this field [5, 35], a major limitation of the present work is selection bias, since all the included patients had failed a previous SBT and were monitored with a Swan-Ganz catheter.
Respiratory failure was considered to be associated with LVHF if PAOP reached at least 18 mmHg. Measures of PAOP above 18 mmHg suggest a hydrostatic cause of pulmonary edema, and although this threshold is used to differentiate pulmonary edema [16] from acute respiratory distress syndrome [36], several issues should be raised. The PAOP may be higher than 18 mmHg without hydrostatic edema due to elevations in pleural pressure generated by external PEEP or by PEEPi [37]. Patients with a chronically elevated PAOP (as seen in patients with valvular heart diseases) do not show the classical clinical signs and symptoms of acute pulmonary edema. Moreover, pleural pressure increases when expiratory muscles contract, and it is difficult to determine PAOP in this scenario [38]. Finally, we did not determine the real transmural PAOP [39, 40].
The Swan-Ganz catheter is a reference method to identify the onset of LVHF during weaning from mechanical ventilation [5, 34], but this is an invasive method and does not allow to determine the mechanism of LVHF. In contrast, echocardiography could detect diastolic heart failure when an episode of LVHF occurs in patients with a normal systolic function [5, 31, 32].
Although we found a negative cumulative fluid balance immediately before the study, we cannot exclude the possibility that the increase in PAOP was related to hypervolemia. It has been shown that a positive fluid balance was associated with failure to wean and with a higher risk of extubation failure [41, 42]. Our data actually suggest that fluid overload probably played a role since the cumulative fluid balance was significantly more negative on the extubation day than on the study day (Table 4). We did not systematically look for other potential causes of SBT failure, such as muscle weakness or critical illness-associated polyneuropathy. Our study is also limited by the fact that our clinical suspicion of diastolic heart failure (because of plausible clinical findings and symptoms, and a rapid response to vasodilators and diuretics) could not be assessed with echocardiographic examination.
Finally, we did not extubate the patients who succeeded a PSV trial. We did not do this because it has been shown that a spontaneous breathing trial using T-piece mimics the WOB performed after extubation well [43], and an extubation failure is associated with high mortality [44]. Nevertheless, we acknowledge that selected patients might benefit from noninvasive ventilation after a risky extubation [45, 46].
Conclusion
In this selected population of difficult-to-wean subjects, the patients’ clinical and physiological behavior differed markedly when a confirmatory SBT was carried out with different techniques. The addition of ventilator support, in terms of PSV or PSV plus PEEP, profoundly modified the breathing pattern, inspiratory muscle effort, and the cardiovascular response when compared to a T-piece trial. Whether we should extubate difficult-to-wean patients who do not tolerate a confirmatory T-piece trial but do tolerate low levels of PSV without PEEP remains unsolved.
References
Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, Johnson MM, Browder RW, Bowton DL, Haponik EF (1996) Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 335:1864–1869
Tobin MJ, Jubran A (2006) Variable performance of weaning-predictor tests: role of Bayes’ theorem and spectrum and test-referral bias. Intensive Care Med 32:2002–2012
Buda AJ, Pinsky MR, Ingels NB Jr, Daughters GT 2nd, Stinson EB, Alderman EL (1979) Effect of intrathoracic pressure on left ventricular performance. N Engl J Med 301:453–459
Lemaire F, Teboul JL, Cinotti L, Giotto G, Abrouk F, Steg G, Macquin-Mavier I, Zapol WM (1988) Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology 69:171–179
Lamia B, Maizel J, Ochagavia A, Chemla D, Osman D, Richard C, Teboul JL (2009) Echocardiographic diagnosis of pulmonary artery occlusion pressure elevation during weaning from mechanical ventilation. Crit Care Med 37:1696–1701
Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, Gasparetto A, Lemaire F (1994) Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med 150:896–903
Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, Fernandez R, de la Cal MA, Benito S, Tomas R et al (1995) A comparison of four methods of weaning patients from mechanical ventilation Spanish Lung Failure Collaborative Group. N Engl J Med 332:345–350
Esteban A, Alia I, Gordo F, Fernandez R, Solsona JF, Vallverdu I, Macias S, Allegue JM, Blanco J, Carriedo D, Leon M, de la Cal MA, Taboada F, Gonzalez de Velasco J, Palazon E, Carrizosa F, Tomas R, Suarez J, Goldwasser RS (1997) Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med 156:459–465
Ezingeard E, Diconne E, Guyomarc’h S, Venet C, Page D, Gery P, Vermesch R, Bertrand M, Pingat J, Tardy B, Bertrand JC, Zeni F (2006) Weaning from mechanical ventilation with pressure support in patients failing a T-tube trial of spontaneous breathing. Intensive Care Med 32:165–169
Sassoon CS, Light RW, Lodia R, Sieck GC, Mahutte CK (1991) Pressure-time product during continuous positive airway pressure, pressure support ventilation, and T-piece during weaning from mechanical ventilation. Am Rev Respir Dis 143:469–475
Koh Y, Hong SB, Lim CM, Lee SD, Kim WS, Kim DS, Kim WD (2000) Effect of an additional 1-hour T-piece trial on weaning outcome at minimal pressure support. J Crit Care 15:41–45
Kuhlen R, Max M, Dembinski R, Terbeck S, Jurgens E, Rossaint R (2003) Breathing pattern and workload during automatic tube compensation, pressure support and T-piece trials in weaning patients. Eur J Anaesthesiol 20:10–16
Cabello B, Mancebo J (2006) Cardiovascular and Respiratory Alterations during Different Spontanous Breathing Trials The role of congestive heart failure. Am J Respir Crit Care Med 3:A40
Girault C, Breton L, Richard JC, Tamion F, Vandelet P, Aboab J, Leroy J, Bonmarchand G (2003) Mechanical effects of airway humidification devices in difficult to wean patients. Crit Care Med 31:1306–1311
Vallverdu I, Calaf N, Subirana M, Net A, Benito S, Mancebo J (1998) Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med 158:1855–1862
Fishman A (1985) Pulmonary Circulation Handbook of physiology. The respiratory system Vol I. Circulation and nonrespiratory functions. American Physiological Society, Bethesda, pp 93–166
Baydur A, Behrakis PK, Zin WA, Jaeger MJ, Milic-Emili J (1982) A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126:788–791
Cabello B, Mancebo J (2006) Work of breathing. Intensive Care Med 32:1311–1314
Mancebo J, Albaladejo P, Touchard D, Bak E, Subirana M, Lemaire F, Harf A, Brochard L (2000) Airway occlusion pressure to titrate positive end-expiratory pressure in patients with dynamic hyperinflation. Anesthesiology 93:81–90
Fleury B, Murciano D, Talamo C, Aubier M, Pariente R, Milic Emili J (1985) Work of breathing in patients with chronic obstructive pulmonary disease in acute respiratory failure. Am Rev Respir Dis 131:822–827
Brochard L, Harf A, Lorino H, Lemaire F (1989) Inspiratory pressure support prevents diaphragmatic fatigue during weaning from mechanical ventilation. Am Rev Respir Dis 139:513–521
El-Khatib MF, Zeineldine SM, Jamaleddine GW (2008) Effect of pressure support ventilation and positive end expiratory pressure on the rapid shallow breathing index in intensive care unit patients. Intensive Care Med 34:505–510
Brochard L, Pluskwa F, Lemaire F (1987) Improved efficacy of spontaneous breathing with inspiratory pressure support. Am Rev Respir Dis 136:411–415
Epstein SK, Ciubotaru RL (1996) Influence of gender and endotracheal tube size on preextubation breathing pattern. Am J Respir Crit Care Med 154:1647–1652
Smith TC, Marini JJ (1988) Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction. J Appl Physiol 65:1488–1499
Richard C, Teboul JL, Archambaud F, Hebert JL, Michaut P, Auzepy P (1994) Left ventricular function during weaning of patients with chronic obstructive pulmonary disease. Intensive Care Med 20:181–186
Lenique F, Habis M, Lofaso F, Dubois-Rande JL, Harf A, Brochard L (1997) Ventilatory and hemodynamic effects of continuous positive airway pressure in left heart failure. Am J Respir Crit Care Med 155:500–505
Pierard LA, Lancellotti P (2004) The role of ischemic mitral regurgitation in the pathogenesis of acute pulmonary edema. N Engl J Med 351:1627–1634
Jubran A, Tobin MJ (1997) Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med 155:906–915
Jubran A, Mathru M, Dries D, Tobin MJ (1998) Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof. Am J Respir Crit Care Med 158:1763–1769
Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, Ghali JK, Liebson PR (2001) Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 104:779–782
Zile MR, Baicu CF, Gaasch WH (2004) Diastolic heart failure-abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350:1953–1959
Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM, Little WC (2001) The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 344:17–22
Richard C, Teboul JL (2005) Weaning failure from cardiovascular origin. Intensive Care Med 31:1605–1607
Zakynthinos S, Routsi C, Vassilakopoulos T, Kaltsas P, Zakynthinos E, Kazi D, Roussos C (2005) Differential cardiovascular responses during weaning failure: effects on tissue oxygenation and lactate. Intensive Care Med 31:1634–1642
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R (1994) The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Pinsky MR (2003) Clinical significance of pulmonary artery occlusion pressure. Intensive Care Med 29:175–178
Hoyt JD, Leatherman JW (1997) Interpretation of the pulmonary artery occlusion pressure in mechanically ventilated patients with large respiratory excursions in intrathoracic pressure. Intensive Care Med 23:1125–1131
Feihl F, Broccard AF (2009) Interactions between respiration and systemic hemodynamics, part I: basic concepts. Intensive Care Med 35:45–54
Feihl F, Broccard AF (2009) Interactions between respiration and systemic hemodynamics, part II: practical implications in critical care. Intensive Care Med 35:198–205
Frutos-Vivar F, Ferguson ND, Esteban A, Epstein SK, Arabi Y, Apezteguía C, González M, Hill NS, Nava S, D’Empaire G, Anzueto A (2006) Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest 130:1664–1671
Upadya A, Tilluckdharry L, Muralidharan V, Amoateng-Adjepong Y, Manthous CA (2005) Fluid balance and weaning outcomes. Intensive Care Med 31:1643–1647
Straus C, Louis B, Isabey D, Lemaire F, Harf A, Brochard L (1998) Contribution of the endotracheal tube and the upper airway to breathing workload. Am J Respir Crit Care Med 157:23–30
Epstein SK, Ciubotaru RL, Wong JB (1997) Effect of failed extubation on the outcome of mechanical ventilation. Chest 112:186–192
Nava S, Gregoretti C, Fanfulla F, Squadrone E, Grassi M, Carlucci A, Beltrame F, Navalesi P (2005) Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med 33:2465–2470
Ferrer M, Sellares J, Valencia M, Carrillo A, Gonzalez G, Badia JR, Nicolas JM, Torres A (2009) Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 374:1082–1088
Acknowledgments
B.C. was supported by grants from the Instituto de Salud Carlos III (expedient CM04/00096, Ministerio de Sanidad) and the Instituto de Recerca Hospital de la Santa Creu i Sant Pau.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cabello, B., Thille, A.W., Roche-Campo, F. et al. Physiological comparison of three spontaneous breathing trials in difficult-to-wean patients. Intensive Care Med 36, 1171–1179 (2010). https://doi.org/10.1007/s00134-010-1870-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1870-0