Abstract
Objective
Weaning-induced cardiogenic pulmonary oedema is a cause of weaning failure that is classically diagnosed by an increase in pulmonary artery occlusion pressure during a spontaneous breathing trial. During cardiogenic pulmonary oedema, a hypo-oncotic fluid is filtered toward the interstitial space. Thus, we tested whether the changes in plasma protein concentration during a weaning trial could diagnose weaning-induced pulmonary oedema.
Design
Prospective study.
Setting
The 24-bed medical intensive care unit of a university hospital.
Patients
Forty-six patients who had failed two weaning trials.
Intervention
Weaning-induced pulmonary oedema was diagnosed if a respiratory failure associated with an increase in pulmonary artery occlusion pressure above 18 mmHg occurred during a third weaning trial on a T-tube. The plasma protein concentration was measured before and at the end of the spontaneous breathing trial.
Measurements and results
During the weaning trial, pulmonary oedema was observed in 24 patients. In these patients, the plasma protein concentration increased by 11% (3–25%). The plasma protein concentration did not change significantly in patients who did not experience weaning-induced pulmonary oedema. An increase in the plasma protein concentration greater than 6% from baseline to the end of the weaning trial allowed detecting a weaning-induced pulmonary oedema with a sensitivity of 87% and a specificity of 95%.
Conclusion
The acute changes in plasma protein concentration during a weaning trial represent an alternative method to right heart catheterisation for assessing weaning-induced pulmonary oedema.
Similar content being viewed by others
Introduction
Failing at weaning from mechanical ventilation leads to prolonged intubation with its inherent risks of increased morbidity and mortality [1]. Identification of the causes of weaning failure is important because it could enable specific therapy and perhaps shorten the delay until extubation.
Among the causes of weaning failure, weaning-induced cardiogenic pulmonary oedema is important to detect, since administration of vasodilators/diuretics may prevent its onset and may enable prompt extubation [2]. Demonstration of weaning-induced pulmonary oedema in a patient who is failing at a weaning trial is often a challenge [3]. To date, right heart catheterisation remains the reference diagnostic method, showing an increase in the pulmonary artery occlusion pressure (PAOP) during a weaning trial [4, 5]. Right heart catheterisation, however, is invasive, and in the particular context of weaning, measuring the PAOP during respiratory failure can be very difficult for non-experts [6]. Simpler diagnostic tools are thus needed in this context.
Hydrostatic pulmonary oedema is accompanied by transfer of a hypo-oncotic fluid from the pulmonary capillaries' lumen toward the interstitium [7–12]. When the amount of transferred fluid is large enough [12], hydrostatic pulmonary oedema may result in haemoconcentration that can be detected on the basis of changes in plasma protein or haemoglobin concentrations or haematocrit [10–12]. We hypothesised that an abrupt occurrence of haemoconcentration during weaning may help to diagnose weaning-induced pulmonary oedema as defined by intolerance to spontaneous breathing and increase in PAOP to a value above 18 mmHg at the end of a weaning trial.
Patients and methods
Patients
From June 2005 to January 2007, we prospectively studied 46 patients who had already failed two successive weaning trials. The screening criteria for performing a weaning trial, as well as weaning trial modalities and the definition of weaning failure that we use in our unit, are detailed in the ESM.
Exclusion criteria were neuromuscular disease, tracheostomy or a contra-indication to right heart catheterisation. According to our practice, a pulmonary artery catheter (CCOmboV; Edwards Lifesciences, Irvine, CA) was inserted after the second failed weaning trial to detect weaning-induced pulmonary oedema. A third weaning trial was performed on the next day.
The ethics committee of the Société de Réanimation de Langue Française approved the study and considered it as part of routine practice. The requirement for informed consent was waived, but all patients received written information about the study as requested by the ethics committee.
Data collected
Before performing the third weaning trial we recorded heart rate, systemic arterial pressure, pulmonary artery pressure, PAOP (at end expiration), cardiac index (bolus thermodilution), mixed venous oxygen saturation, arterial blood gases, plasma protein and arterial haemoglobin concentrations. Left ventricular ejection fraction was measured by echocardiography.
A second set of measurements was performed at the end of the trial, i. e. just before extubation in patients who succeeded at the 60-min trial or just before reconnection to the ventilator in the patients who could not tolerate spontaneous breathing. Weaning-induced pulmonary oedema was diagnosed (1) if patients exhibited signs of weaning failure (see above) and (2) if the PAOP at the end of the weaning trial was ≥ 18 mmHg [13].
The plasma protein concentration was measured with the Modular P device (Roche Diagnostics, Basel, Switzerland). The repeatability of plasma protein concentration measurements with this device is reported (by the manufacturer) to be 0.6 ± 0.9%. Haemoglobin concentration was measured from the arterial blood sample by means of two different devices: the IL Synthesis25 (Instrumentation Laboratory, Lexington, MA) from June 2005 to January 2006, and the ABL 800flex (Radiometer, Copenhagen, Denmark) from February 2006 to January 2007. The repeatability of the haemoglobin concentration measurements with the ABL 800flex is reported (by the manufacturer) to be 0.6 ± 1.4%.
Statistical analysis
Continuous variables were expressed as median (range). Comparisons between before vs. end of the weaning trial were assessed using a paired Student t-test. Comparisons of variables and comparisons of weaning-trial-induced changes in variables between patients with and patients without weaning-induced pulmonary oedema were assessed using a two-sample Student t-test. Non-continuous dichotomous data were compared using the X 2 test with Yates correction or Fisher's exact test as appropriate. For all variables that were found to be statistically different between patients with and without weaning-induced pulmonary oedema, receiver operating characteristic (ROC) curves were generated to test their ability to diagnose weaning-induced pulmonary oedema. The areas under these ROC curves ( ± SE) were compared using a Hanley–McNeil test. The positive and negative likelihood ratios for the diagnosis of weaning-induced pulmonary oedema were calculated. A p-value ≤ 0.05 was considered statistically significant. The analysis was performed using Statview 5.0 (Abacus concepts, Berkeley, CA) and MedCalc8.1.0.0 (Mariakerke, Belgium) software.
Results
Outcome of the weaning trial
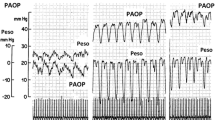
According to our definition, we identified 24 patients with and 22 patients without weaning-induced pulmonary oedema (Table 1). During the weaning trial and compared to patients without weaning-induced pulmonary oedema, patients with weaning-induced pulmonary oedema exhibited significantly larger increases in PAOP (Fig. 1), heart rate, systolic, diastolic and mean systemic arterial pressure, systolic, diastolic and mean pulmonary artery pressure, respiratory rate and PaCO2 (Table 2).
The duration of the weaning trial was 28 (5–60) min and 45 (15–60) min in patients with and without weaning-induced pulmonary oedema, respectively (p = 0.01). A significantly larger proportion of patients with than without weaning-induced pulmonary oedema failed at the weaning trial (100% vs. 47%). All the patients who succeeded at the weaning trial (n = 12) were successfully extubated during the next hour. In patients without pulmonary oedema, the weaning failure was attributed to the severity of a chronic respiratory failure in nine cases, to an underlying pneumonia in five cases, to a neuromyopathy in two cases and remained unknown in six cases.
Ability of changes in plasma protein concentration to detect weaning-induced pulmonary oedema
During the weaning trial, plasma protein concentration did not change significantly in patients without weaning-induced pulmonary oedema, while it increased significantly by 11% (3–25%) in patients with weaning-induced pulmonary oedema (Fig. 1). The absolute changes in plasma protein concentration during the weaning trial were greater in patients with than without weaning-induced pulmonary oedema [6 (2–13) g/l vs. 2 (0–5) g/l, respectively]. An increase in plasma protein concentration greater than 6.0% during the weaning trial detected weaning-induced pulmonary oedema with a sensitivity of 87% (95% CI 66–99%) and a specificity of 95% (95% CI 77–99%) (Fig. 2). The Youden index was 0.85. This 6.0% increase cut-off provided a high positive likelihood ratio value (19.25) and a negative likelihood ratio value of 0.13. A positive likelihood ratio > 10 and a negative likelihood ratio < 0.1 were provided by an increase in plasma protein concentration > 5.0 % (see ESM). The ROC curve generated for the percentage changes in plasma protein concentration was of significantly larger area than that generated for any other variable found to differ between patients with and without weaning-induced pulmonary oedema (Table 3).
Ability of changes in haemoglobin concentration to detect weaning-induced pulmonary oedema
During the weaning trial, haemoglobin concentration did not change significantly in patients without weaning-induced pulmonary oedema, while it significantly increased by 10% (2–41%) in patients with weaning-induced pulmonary oedema (Table 2). The absolute changes in haemoglobin concentration in weaning-induced pulmonary oedema patients were not significantly different between patients with- and without weaning-induced pulmonary oedema. An increase of haemoglobin concentration > 6.7% during the weaning trial detected weaning-induced pulmonary oedema with a sensitivity of 71% (95% CI 49–87%) and a specificity of 86% (95% CI 64–95%). The Youden index was 0.52. A positive likelihood ratio > 10 was provided by an increase in haemoglobin concentration > 8.8%, and a negative likelihood ratio < 0.1 was provided by an increase in haemoglobin concentration < 1.0% (see ESM). The ROC curve generated for the changes in haemoglobin concentration was of significantly smaller area than that generated for the changes in plasma protein concentration (Table 3, Fig. 3). A combined criterion (increase in plasma protein concentration > 6.0% and increase in haemoglobin concentration > 6.7%) displayed sensitivity of 87% and specificity of 100% for diagnosing weaning-induced pulmonary oedema.
However, taking into account only the 32 patients in whom the haemoglobin concentration was measured with the ABL 800flex device, an increase in haemoglobin concentration ≥ 6.5% diagnosed weaning-induced pulmonary oedema with a sensitivity of 93% (95% CI 68–99%) and a specificity of 77% (95% CI 50–93%). In these patients, the changes in haemoglobin and in plasma protein concentration were correlated (r = 0.70, p < 0.05) and the area under the ROC curves generated for the weaning-induced changes in plasma protein concentration and in haemoglobin concentration were not statistically different (0.99 ± 0.01 and 0.88 ± 0.06, respectively).
The 24 patients with weaning-induced pulmonary oedema received treatment after the weaning trial. All received diuretics, eight received nitrates, four received angiotensin-converting enzyme inhibitors and two received verapamil. Two patients eventually died under mechanical ventilation. All others were eventually extubated 4 (1–20) days later. Between the third and the fourth weaning trials in these patients, the fluid balance was −5,000 (−1,000–20,000) ml. In 13 patients the fourth weaning trial was performed while the pulmonary artery catheter was still in place. None of these patients experienced a new episode of weaning-induced pulmonary oedema. Indeed, in this subgroup, PAOP was 9 (6–16) mmHg before and 14 (7–17) mmHg at the end of the fourth weaning trial, while at the third trial PAOP had increased from 12 (7–16) mmHg to 28 (19–35) mmHg (p < 0.01). Plasma protein concentration did not change at the fourth weaning trial in these 13 patients [from 60 (46–70) g/l to 60 (54–64) g/l, p = 0.83], while it had increased from 56 (41–66) g/l to 60 (50–74) g/l (p = 0.05) at the third weaning trial.
Discussion
The present study demonstrates that an increase in plasma protein concentration by more than 6% during a weaning trial could detect the onset of weaning-induced pulmonary oedema with sensitivity of 87% and specificity of 95%.
In our population of difficult-to-wean patients, we identified a high proportion with weaning-induced cardiogenic pulmonary oedema. As detailed elsewhere [14], several mechanisms can be involved in the development of cardiogenic pulmonary oedema during weaning from mechanical ventilation. Increase in left ventricular afterload [15] and increase in venous return and cardiac preload [5] during weaning may contribute to increasing central blood volume. In some predisposed patients, right ventricle enlargement may also occur, resulting in reduced left ventricular compliance associated with increased left ventricular filling pressure [5]. The fact that the plasma protein concentration was lower in patients of our study who exhibited pulmonary oedema at weaning suggests that they were relatively more overloaded than the patients who did not experience weaning-induced pulmonary oedema. In addition, disconnecting the patient from the ventilator can result in a marked increase in work of breathing and in adrenergic state [5] and hence in myocardial oxygen demand, with the potential risk of myocardial ischaemia in the case of prior coronary artery disease [16–19]. Since the classical signs of pulmonary oedema such as crackles and foamy expectorations are usually lacking in this situation, the confident diagnosis of weaning-induced pulmonary oedema is based upon the assessment of an increase in PAOP above 18 mmHg [2], as we used in the present study as gold standard. However, the interpretation of the PAOP trace during marked inspiratory efforts and large changes in intrathoracic pressure could be particularly difficult for non-expert practitioners [6]. In addition, physicians could be reluctant to insert a pulmonary artery catheter at the time of weaning, when invasive devices have usually been removed. Thus, alternative non-invasive methods are needed for identifying weaning-induced pulmonary oedema.
The striking result of our study was that an increase in plasma protein concentration during the weaning trial was able adequately to identify patients with weaning-induced pulmonary oedema. Interestingly, in 13 patients who experienced a weaning-induced pulmonary oedema at the third trial, the fourth weaning trial was monitored with a pulmonary artery catheter after they had received diuretics and/or vasodilators. Among these patients, who could be considered as their own controls, none experienced a recurrent weaning-induced pulmonary oedema and the plasma protein concentration no longer increased during the fourth trial. During cardiogenic pulmonary oedema, the increase in the pulmonary capillary pressure favours the filtration of fluid toward the alveolo-capillary barrier. Provided that the integrity of the capillary membrane is preserved, the fluid that egresses from the intravascular compartment toward the interstitium is hypo-oncotic with respect to plasma [7–12]. It has been demonstrated that the oedema fluid has approximately one-half of the protein content of plasma [8, 9]. Since the amount of filtered fluid may be large, haemoconcentration should ensue, as has been demonstrated in animals [20] and in patients developing acute cardiogenic pulmonary oedema. Such haemoconcentration has been shown to reverse when patients improved with therapy [10–12]. The 11% average increase in plasma protein concentration we observed in our patients with weaning-induced pulmonary oedema was in accordance with the range of plasma protein concentration decrease (−10% to −18%) observed by Figueiras and Weil [10, 11] during resolution of cardiogenic pulmonary oedema. The 11% increase in plasma protein concentration observed in our study corresponds to the leak of 500–600 ml of plasma out of the vessels, an amount compatible with what was previously observed during acute pulmonary oedema [12]. As a first clinical application of this physiological concept, Bahloul and colleagues demonstrated the ability of an increase in plasma protein concentration to detect acute cardiogenic pulmonary oedema due to scorpion envenomation [21]. The present study may extend to another form of cardiogenic pulmonary oedema the diagnostic significance of this biological tool.
The specificity of the increase in plasma protein concentration for diagnosing weaning-induced pulmonary oedema was high. In fact, it is difficult to consider other pathophysiological hypotheses than sudden hydrostatic pulmonary oedema development to explain such abrupt changes in plasma protein concentration: neither the total amount of plasma protein nor the plasma volume in which proteins are diluted may have changed over such a short time. The sensitivity of the method was slightly lower, since we identified four patients in whom plasma protein concentration changed by a small amount although weaning-induced pulmonary oedema was evidenced by a marked weaning-induced increase in PAOP, which ranged from 22 to 32 mmHg at the end of the weaning trial. In three of these patients, the changes in plasma protein concentration were close to the 6% cut-off value but the changes in haemoglobin concentration were higher than 6%, such that they were well classified by the haemoglobin method. The remaining false-negative patient had a low increase in plasma protein concentration as well as in haemoglobin concentration, and we found no evident reason for this diagnostic failure. We observed that changes in plasma protein concentration performed better than those in haemoglobin concentration for establishing the diagnosis of weaning-induced pulmonary oedema. One explanation could be that the device used for measuring the haemoglobin concentration during the earlier period of the study (in the first 14 patients) was less accurate than the one we used later. The diagnostic value of the changes in haemoglobin concentration improved greatly when considering only the 32 patients who benefited from the ABL 800flex method, and the correlation between changes in haemoglobin and in plasma protein concentration in this population was high. Nonetheless, when considering the use of both changes in plasma protein and changes in haemoglobin concentration to detect haemoconcentration, only one false-negative and two false-positive cases were observed.
We acknowledge several limitations of our study. First, the diagnosis of pulmonary oedema was based upon a high value of PAOP combined with evident respiratory distress, as previously reported [13]. We are aware that the diagnosis of pulmonary oedema should be based upon assessment of the extravascular lung water rather than on measurement of PAOP, since PAOP can be low in the case of non-hydrostatic pulmonary oedema. Nevertheless, in the context of the present study, the problem was to demonstrate the haemodynamic trigger of pulmonary oedema during weaning (namely, the increase in left ventricular filling pressure), and for this purpose the measurement of PAOP seemed particularly appropriate, although the classical cut-off of 18 mmHg [13] could be a matter of debate. In our study, we did not measure biomarkers of cardiac dysfunction to diagnose weaning-induced pulmonary oedema. Nevertheless, in one previous study the time-course of the value of B-type natriuretic peptide (from the start to the end of a weaning trial) did not enable accurate differentiation of patients who succeeded from patients who failed the trial [22]. In a recent study, N-terminal pro-brain natriuretic peptide was found to detect weaning-induced acute cardiac dysfunction in difficult-to-wean patients. However, in that study, the definition of weaning-induced cardiac dysfunction was based only on experts' judgement [23]. Second, the threshold we found for the plasma protein concentration change (6.0%) seems low, suggesting that the diagnosis may be subject to collection or sampling errors. However, this value is far larger than the repeatability of the dosing device provided by the manufacturer (0.6 ± 0.9%) although, as far as we know, no data on this repeatability have been published. Third, the number of patients was relatively small in our study and our results should be confirmed by further studies. Fourth, since in patients with weaning-induced pulmonary oedema diuretics were started immediately after the diagnosis, we could not show the disappearance of haemoconcentration once these patients were connected again to the ventilator. Finally, we used two different devices for measuring the haemoglobin concentration. Further studies should test the diagnostic accuracy of the haemoglobin changes with the most recent device in a larger collective. On the same lines, the diagnostic accuracy of haematocrit, a sensitive marker of haemoconcentration, should also be tested in further studies.
In conclusion, our study demonstrates that weaning-induced pulmonary oedema could be detected by the acute increase in plasma protein concentration measured at the end of the weaning trial. This method could represent a fairly non-invasive alternative to right heart catheterisation in this context.
References
Epstein SK, Ciubotaru RL, Wong JB (1997) Effect of failed extubation on the outcome of mechanical ventilation. Chest 112:186–192
Richard C, Teboul JL (2005) Weaning failure from cardiovascular origin. Intensive Care Med 31:1605–1607
Monnet X, Teboul JL, Richard C (2007) Cardiopulmonary interactions in patients with heart failure. Curr Opin Crit Care 13:6–11
Jubran A, Mathru M, Dries D, Tobin MJ (1998) Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof. Am J Respir Crit Care Med 158:1763–1769
Lemaire F, Teboul JL, Cinotti L, Giotto G, Abrouk F, Steg G, Macquin-Mavier I, Zapol WM (1988) Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology 69:171–179
Pinsky MR (2003) Pulmonary artery occlusion pressure. Intensive Care Med 29:19–22
Sedziwy L, Thomas M, Shillingford J (1968) Some observations on haematocrit changes in patients with acute myocardial infarction. Br Heart J 30:344–349
Vreim CE, Snashall PD, Staub NC (1976) Protein composition of lung fluids in anesthetized dogs with acute cardiogenic edema. Am J Physiol 231:1466–1469
Fein A, Grossman RF, Jones JG, Overland E, Pitts L, Murray JF, Staub NC (1979) The value of edema fluid protein measurement in patients with pulmonary edema. Am J Med 67:32–38
Figueras J, Weil MH (1977) Increases in plasma oncotic pressure during acute cardiogenic pulmonary edema. Circulation 55:195–199
Figueras J, Weil MH (1978) Blood volume prior to and following treatment of acute cardiogenic pulmonary edema. Circulation 57:349–355
Henning RJ, Weil MH (1978) Effect of afterload reduction on plasma volume during acute heart failure. Am J Cardiol 42:823–827
McHugh TJ, Forrester JS, Adler L, Zion D, Swan HJ (1972) Pulmonary vascular congestion in acute myocardial infarction: hemodynamic and radiologic correlations. Ann Intern Med 76:29–33
Lamia B, Monnet X, Teboul JL (2005) Weaning- induced cardiac dysfunction. In: Vincent JL (ed) Yearbook of intensive care and emergency medicine. Springer, Berlin, pp 239–245
Richard C, Teboul JL, Archambaud F, Hebert JL, Michaut P, Auzepy P (1994) Left ventricular function during weaning of patients with chronic obstructive pulmonary disease. Intensive Care Med 20:181–186
Hurford WE, Favorito F (1995) Association of myocardial ischemia with failure to wean from mechanical ventilation. Crit Care Med 23:1475–1480
Hurford WE, Lynch KE, Strauss HW, Lowenstein E, Zapol WM (1991) Myocardial perfusion as assessed by thallium-201 scintigraphy during the discontinuation of mechanical ventilation in ventilator-dependent patients. Anesthesiology 74:1007–1016
Srivastava S, Chatila W, Amoateng-Adjepong Y, Kanagasegar S, Jacob B, Zarich S, Manthous CA (1999) Myocardial ischemia and weaning failure in patients with coronary artery disease: an update. Crit Care Med 27:2109–2112
Chatila W, Ani S, Guaglianone D, Jacob B, Amoateng-Adjepong Y, Manthous CA (1996) Cardiac ischemia during weaning from mechanical ventilation. Chest 109:1577–1583
Weiser PC, Grande F (1974) Estimation of fluid shifts and protein permeability during pulmonary edemagenesis. Am J Physiol 226:1028–1034
Bahloul M, Bouaziz M, Dammak H, Ben Hamida C, Ksibi H, Rekik N, Chelly H, Teboul JL, Kallel H (2002) Value of the plasma protein and hemoglobin concentration in the diagnosis of pulmonary edema in scorpion sting patients. Intensive Care Med 28:1600–1605
Mekontso-Dessap A, de Prost N, Girou E, Braconnier F, Lemaire F, Brun-Buisson C, Brochard L (2006) B-type natriuretic peptide and weaning from mechanical ventilation. Intensive Care Med 32:1529–1536
Grasso S, Leone A, De Michele M, Anaclerio R, Cafarelli A, Ancona G, Stripoli T, Bruno F, Pugliese P, Dambrosio M, Dalfino L, Di Serio F, Fiore T (2007) Use of N-terminal pro-brain natriuretic peptide to detect acute cardiac dysfunction during weaning failure in difficult-to-wean patients with chronic obstructive pulmonary disease. Crit Care Med 35:96–105
Author information
Authors and Affiliations
Corresponding author
Additional information
N. Anguel and X. Monnet contributed equally to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Anguel, N., Monnet, X., Osman, D. et al. Increase in plasma protein concentration for diagnosing weaning-induced pulmonary oedema. Intensive Care Med 34, 1231–1238 (2008). https://doi.org/10.1007/s00134-008-1038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1038-3