Abstract
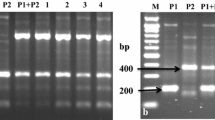
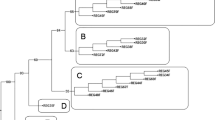
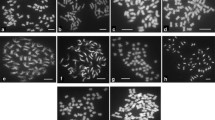
The somatic hybrids were derived previously from protoplast fusion between Solanum tuberosum and S. chacoense to gain the bacterial wilt resistance from the wild species. The genome components analysis in the present research was to clarify the nuclear and cytoplasmic composition of the hybrids, to explore the molecular markers associated with the resistance, and provide information for better use of these hybrids in potato breeding. One hundred and eight nuclear SSR markers and five cytoplasmic specific primers polymorphic between the fusion parents were used to detect the genome components of 44 somatic hybrids. The bacterial wilt resistance was assessed thrice by inoculating the in vitro plants with a bacterial suspension of race 1. The disease index, relative disease index, and resistance level were assigned to each hybrid, which were further analyzed in relation to the molecular markers for elucidating the potential genetic base of the resistance. All of the 317 parental unique nuclear SSR alleles appeared in the somatic hybrids with some variations in the number of bands detected. Nearly 80 % of the hybrids randomly showed the chloroplast pattern of one parent, and most of the hybrids exhibited a fused mitochondrial DNA pattern. One hundred and nine specific SSR alleles of S. chacoense were analyzed for their relationship with the disease index of the hybrids, and three alleles were identified to be significantly associated with the resistance. Selection for the resistant SSR alleles of S. chacoense may increase the possibility of producing resistant pedigrees.



Similar content being viewed by others
References
Babiychuk E, Kushnir S, Gleba YY (1992) Spontaneous extensive chromosome elimination in somatic hybrids between somatically congruent species Nicotiana tabacum L. and Atropa belladonna L. Theor Appl Genet 84:87–91
Bastia T, Carotenuto N, Basile B, Zoina A, Cardi T (2000) Induction of novel organelle DNA variation and transfer of resistance to frost and verticillium wilt in Solanum tuberosum through somatic hybridization with 1EBN S. commersonii. Euphytica 116:1–10
Bidani A, Nouri-Ellouz O, Lakhoua L, Sihachakr D, Cheniclet C, Mahjoub A, Drira N, Gargouri-Bouzid R (2007) Interspecific potato somatic hybrids between Solanum berthaultii and Solanum tuberosum L. showed recombinant plastome and improved tolerance to salinity. Plant Cell Tiss Org 91:179–189
Boltowicz D, Szczerbakowa A, Wielgat B (2005) RAPD analysis of the interspecific somatic hybrids: solanum bulbocastanum (+) S. tuberosum. Cell Mol Biol Lett 10:151–162
Cai XK, Liu J, Xie CH (2004) Mesophyll protoplast fusion of Solanum tuberosum and Solanum chacoense and their somatic hybrid analysis. Acta Hortic Sin 31:623–626
Cardi T, Bastia T, Monti L, Earle ED (1999) Organelle DNA and male fertility variation in Solanum spp. and interspecific somatic hybrids. Theor Appl Genet 99:819–828
Collonnier C, Mulya K, Fock I et al (2001) Source of resistance against Ralstonia solanacearum in fertile somatic hybrids of eggplant (Solanum melongena L) with Solanum aethiopicum L. Plant Sci 160:301–313
Davey MR, Anthony P, Power JB, Lowe KC (2005) Plant protoplasts: status and biotechnological perspectives. Biotechnol Adv 23:131–171
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS (1991) “Touchdown” PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res 19:4008
Feingold S, Lloyd J, Norero N, Bonierbale M, Lorenzen J (2005) Mapping and characterization of new EST-derived microsatellites for potato (Solanum tuberosum L). Theor Appl Genet 111:456–466
Fock I, Collonnier C, Purwito A, Luisetti J, Souvannavong V, Vedel F, Servaes A, Ambroise A, Kodja H, Ducreux G (2001) Use of Solanum stenotomum for introduction of resistance to bacterial wilt in somatic hybrids of potato. Plant Physiol Biochem 39:899–908
Gao G, Qu DY, Lian Y, Jin LP, Feng LX (2000) Identification molecular markers linked with resistance to bacterial wilt (Ralstonia solanacearum) in diploid potato. Acta Hortic Sin 27:37–41
Ghislain M, Jorge N, Herrera MR, Pignataro J, Guzman F, Bonierbale M, Spooner DM (2009) Robust and highly informative microsatellite-based genetic identity kit for potato. Mol Breed 23:377–388
Goremykin VV, Salamini F, Velasco R, Viola R (2008) Mitochondrial DNA of Vitis vinifera and the issue of rampant horizontal gene transfer. Mol Biol Evol 26:99–110
Greplová M, Polzerová H, Vlastní ková H (2008) Electrofusion of protoplasts from Solanum tuberosum, S. bulbocastanum and S. pinnatisectum. Acta Physiol Plant 30:787–796
Guo XP, Xie CH, Cai XK, Song BT, He L, Liu J (2010) Meiotic behavior of pollen mother cells in relation to ploidy level of somatic hybrids between Solanum tuberosum and S. chacoense. Plant Cell Rep 29:1277–1285
Hawkes JG (1994) Origins of cultivated potatoes and species relationships. In: Bradshaw JE, Mackay GR (eds) Potato genetics. CAB International, Oxford, pp 3–42
Hayward AC (1994) Hosts of Pseudomonas solanacearum. In: Hayward AC, Hartman GL (eds) Bacterial wilt: the disease and its causative agent Pseudomonas solanacearum. CAB International, Wallingford, pp 9–23
Hayward AC, Elphinstone JG, Caffier D et al (1998) Round table on bacterial wilt (brown rot) of potato. In: Prior PH, Allen C, Elphinstone JG (eds) Bacterial wilt disease: molecular and ecological aspects. Springer, Heidlberg, pp 420–430
Heinhorst S, Gannon GC, Galun E, Kenschaft L, Weissbach A (1988) Clone bank and physical and genetic map of potato chloroplast DNA. Theor Appl Genet 75:244–251
Hermsen JGT (1994) Introgression of genes from wild species, including molecular and cellular approaches. In: Bradshaw JE, Mackay GR (eds) Potato genetics. CAB International, Cambridge, pp 515–538
Jähne A, Lazzeri PA, Jäger-Gussen M, Lörz H (1991) Plant regeneration from embryogenic cell suspensions derived from anther cultures of barley (Hordeum vulgare L.). Theor Appl Genet 82:74–80
Johnston SA, den Nijs TPM, Peloquin SJ, Hanneman RE Jr (1980) The significance of genic balance to endosperm development in interspecific crosses. Theor Appl Genet 57:5–9
Kawata M, Ohmiya A, Shimamoto Y, Oono K, Takaiwa F (1995) Structural changes in the plastid DNA of rice (Oryza sativa L.) during tissue culture. Theor Appl Genet 90:364–371
Kim-Lee HY, Moon JS, Hong YJ, Kim MS, Cho HM (2005) Bacterial wilt resistance in the progenies of the fusion hybrids between haploid of potato and Solanum commersonii. Am J Potato Res 82:129–137
Kurosaki F, Tsurusawa Y, Nishi A (1987) Breakdown of phosphatidylinositol during the elicitation of phytoalexin production in cultured carrot cells. Plant Physiol 85:601–604
Laferriere LT, Helgeson JP, Allen C (1999) Fertile Solanum tuberosum + S. commersonii somatic hybrids as sources of resistance to bacterial wilt caused by Ralstonia solanacearum. Theor Appl Genet 98:1272–1278
Liang YF, He LY (1999) Detection of the resistance to bacterial wilt in potato (Solanum tuberosum L). Chinese potato 2:109–112
Lössl A, Adler N, Horn R, Frei U, Wenzel G (1999) Chondriome-type characterization of potato: mt α, β, γ, δ, ε and novel plastid-mitochondrial configurations in somatic hybrids. Theor Appl Genet 98:1–10
Lovene M, Savarese S, Cardi T et al (2007) Nuclear and cytoplasmic genome composition of Solanum bulbocastanum (+) S. tuberosum somatic hybrids. Genome 50:443–450
Melin PM, Pical C, Jegril B (1992) Polyphosphoinositide phospholipase C in wheat root plasma membranes. Partial purification and characterization. Biochim Biophys Acta 1123:163–169
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant Physiol 15:473–497
Patel D, Power JB, Anthony P, Badakshi F, Heslop-Harrison JS, Davey MR (2011) Somatic hybrid plant of Nicotiana × sanderae (+) N. debneyi with fungal resistance to Peronospora tabacina. Annals Bot 108:809–819
Pijnacker LP, Ferwerda MA, Puite KJ, Schaart JG (1989) Chromosome elimination and mutation in tetraploid somatic hybrids of Solanum tuberosum and Solanum phureja. Plant Cell Rep 8:82–85
Sarkar D, Tiwari JK, Sharma S, Poonam B, Sharma S, Gopal J, Singh BP, Luthra SK, Pandey SK, Pattanayak D (2011) Production and characterization of somatic hybrids between Solanum tuberosum L. and S. pinnatisectum Dun. Plant Cell Tiss Org 107:427–440
Scotti N, Monti L, Cardi T (2003) Organelle DNA variation in parental Solanum spp. genotypes and nuclear-cytoplasmic interactions in Solanum tuberosum (+) S. commersonii somatic hybrid-backcross progeny. Theor Appl Genet 108:87–94
Scotti N, Cozzolino S, Cardi T (2007) Mitochondrial DNA variation in cultivated and wild potato species (Solanum spp.). Genome 50:706–713
Sugiyama Y, Watase Y, Nagase M et al (2005) The complete nucleotide sequence and multipartite organization of the tobacco mitochondrial genome: comparative analysis of mitochondrial genomes in higher plants. Mol Genet Genomics 272:603–615
Thieme R, Rakosy-Tican E, Nachtigall M et al (2010) Characterization of the multiple resistance traits of somatic hybrids between Solanum cardiophyllum Lindl. and two commercial potato cultivars. Plant Cell Rep 29:1187–1201
Tiwari JK, Poonam B, Sarkar D, Pandey SK, Gopal J, Kumar SR (2010) Molecular and morphological characterization of somatic hybrids between Solanum tuberosum L. and S. etuberosum Lindl. Plant Cell Tiss Org 103:175–187
Trabelsi S, Gargouri-Bouzid R, Vedel F, Nato A, Lakhoua L, Drira N (2005) Somatic hybrids between potato Solanum tuberosum and wild species Solanum vernei exhibit a recombination in the plastome. Plant Cell Tiss Org 83:1–11
Tung PX (1992) Genetic variation for bacterial wilt resistance in a population of tetraploid potato. Euphytica 61:73–80
Winstead NN, Kelman A (1952) Inoculation techniques for evaluating resistance to Pseudomonas solanacearum. Phytopathology 42:628–634
Acknowledgments
The authors thank Professor Jeff Moorby for critical reading and language editing of this manuscript. The work was supported by the Natural Science Foundation of Hubei Province (2009CDA085).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Lightfoot.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, L., Guo, X., Xie, C. et al. Nuclear and cytoplasmic genome components of Solanum tuberosum + S. chacoense somatic hybrids and three SSR alleles related to bacterial wilt resistance. Theor Appl Genet 126, 1861–1872 (2013). https://doi.org/10.1007/s00122-013-2098-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-013-2098-5