Abstract
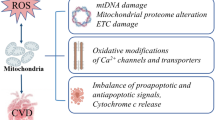
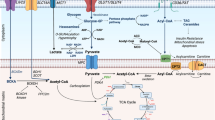
Metabolic cardiomyopathy is an emerging cause of heart failure in patients with obesity, insulin resistance, and diabetes. It is characterized by impaired myocardial metabolic flexibility, intramyocardial triglyceride accumulation, and lipotoxic damage in association with structural and functional alterations of the heart, unrelated to hypertension, coronary artery disease, and other cardiovascular diseases. Oxidative stress plays an important role in the development and progression of metabolic cardiomyopathy. Mitochondria are the most significant sources of reactive oxygen species (ROS) in cardiomyocytes. Disturbances in myocardial substrate metabolism induce mitochondrial adaptation and dysfunction, manifested as a mismatch between mitochondrial fatty acid oxidation and the electron transport chain (ETC) activity, which facilitates ROS production within the ETC components. In addition, non-ETC sources of mitochondrial ROS, such as β-oxidation of fatty acids, may also produce a considerable quantity of ROS in metabolic cardiomyopathy. Augmented ROS production in cardiomyocytes can induce a variety of effects, including the programming of myocardial energy substrate metabolism, modulation of metabolic inflammation, redox modification of ion channels and transporters, and cardiomyocyte apoptosis, ultimately leading to the structural and functional alterations of the heart. Based on the above mechanistic views, the present review summarizes the current understanding of the mechanisms underlying metabolic cardiomyopathy, focusing on the role of oxidative stress.


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH et al (2017) IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50. https://doi.org/10.1016/j.diabres.2017.03.024
Czibik G, d’Humieres T, Derumeaux G (2021) When does too much energy become a danger to the heart? Eur Heart J. https://doi.org/10.1093/eurheartj/ehab801
Ren J, Wu NN, Wang S, Sowers JR, Zhang Y (2021) Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol Rev 101(4):1745–1807. https://doi.org/10.1152/physrev.00030.2020
Zhou J, Bai L, Zhang XJ, Li H, Cai J (2021) Nonalcoholic fatty liver disease and cardiac remodeling risk: pathophysiological mechanisms and clinical implications. Hepatology 74(5):2839–2847. https://doi.org/10.1002/hep.32072
Nishida K, Otsu K (2017) Inflammation and metabolic cardiomyopathy. Cardiovasc Res 113(4):389–398. https://doi.org/10.1093/cvr/cvx012
Maack C, Murphy E (2017) Metabolic cardiomyopathies - fighting the next epidemic. Cardiovasc Res 113(4):367–369. https://doi.org/10.1093/cvr/cvx022
Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO (2013) Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail 1(2):93–102. https://doi.org/10.1016/j.jchf.2013.01.006
Mishra S, Kass DA (2021) Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 18(6):400–423. https://doi.org/10.1038/s41569-020-00480-6
Li J, Li J, Chen Y, Hu W, Gong X, Qiu H et al (2022) The role of mitochondria in metabolic syndrome-associated cardiomyopathy. Oxid Med Cell Longev 2022:9196232. https://doi.org/10.1155/2022/9196232
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30(6):595–602. https://doi.org/10.1016/0002-9149(72)90595-4
Kannel WB, Hjortland M, Castelli WP (1974) Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34(1):29–34. https://doi.org/10.1016/0002-9149(74)90089-7
Jia G, Hill MA, Sowers JR (2018) Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 122(4):624–638. https://doi.org/10.1161/CIRCRESAHA.117.311586
Bugger H, Abel ED (2014) Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57(4):660–671. https://doi.org/10.1007/s00125-014-3171-6
Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M et al (2012) Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest 122(3):1109–1118. https://doi.org/10.1172/JCI60329
Nakamura M, Sadoshima J (2020) Cardiomyopathy in obesity, insulin resistance and diabetes. J Physiol 598(14):2977–2993. https://doi.org/10.1113/JP276747
Mandavia CH, Aroor AR, Demarco VG, Sowers JR (2013) Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci 92(11):601–608. https://doi.org/10.1016/j.lfs.2012.10.028
Cai J, Zhang XJ, Ji YX, Zhang P, She ZG, Li H (2020) Nonalcoholic fatty liver disease pandemic fuels the upsurge in cardiovascular diseases. Circ Res 126(5):679–704. https://doi.org/10.1161/CIRCRESAHA.119.316337
Alpert MA, Karthikeyan K, Abdullah O, Ghadban R (2018) Obesity and cardiac remodeling in adults: mechanisms and clinical implications. Prog Cardiovasc Dis 61(2):114–123. https://doi.org/10.1016/j.pcad.2018.07.012
Jia G, DeMarco VG, Sowers JR (2016) Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 12(3):144–153. https://doi.org/10.1038/nrendo.2015.216
Schilling JD, Machkovech HM, Kim AH, Schwendener R, Schaffer JE (2012) Macrophages modulate cardiac function in lipotoxic cardiomyopathy. Am J Physiol Heart Circ Physiol 303(11):H1366–H1373. https://doi.org/10.1152/ajpheart.00111.2012
Chen Z, Liu J, Zhou F, Li H, Zhang XJ, She ZG et al (2021) Nonalcoholic fatty liver disease: an emerging driver of cardiac arrhythmia. Circ Res 128(11):1747–1765. https://doi.org/10.1161/CIRCRESAHA.121.319059
Bugger H, Abel ED (2008) Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 114(3):195–210. https://doi.org/10.1042/CS20070166
Nicolson GL (2007) Metabolic syndrome and mitochondrial function: molecular replacement and antioxidant supplements to prevent membrane peroxidation and restore mitochondrial function. J Cell Biochem 100(6):1352–1369. https://doi.org/10.1002/jcb.21247
Ilkun O, Boudina S (2013) Cardiac dysfunction and oxidative stress in the metabolic syndrome: an update on antioxidant therapies. Curr Pharm Des 19(27):4806–4817. https://doi.org/10.2174/1381612811319270003
Forman HJ, Zhang H (2021) Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 20(9):689–709. https://doi.org/10.1038/s41573-021-00233-1
Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK (2018) Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122(6):877–902. https://doi.org/10.1161/CIRCRESAHA.117.311401
van der Vliet A, Janssen-Heininger YMW, Anathy V (2018) Oxidative stress in chronic lung disease: from mitochondrial dysfunction to dysregulated redox signaling. Mol Aspects Med 63:59–69. https://doi.org/10.1016/j.mam.2018.08.001
Shah AK, Bhullar SK, Elimban V, Dhalla NS (2021) Oxidative stress as a mechanism for functional alterations in cardiac hypertrophy and heart failure. Antioxidants (Basel) 10(6). https://doi.org/10.3390/antiox10060931
Costantino S, Akhmedov A, Melina G, Mohammed SA, Othman A, Ambrosini S et al (2019) Obesity-induced activation of JunD promotes myocardial lipid accumulation and metabolic cardiomyopathy. Eur Heart J 40(12):997–1008. https://doi.org/10.1093/eurheartj/ehy903
Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L (2020) Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol 17(9):585–607. https://doi.org/10.1038/s41569-020-0339-2
Palomer X, Salvado L, Barroso E, Vazquez-Carrera M (2013) An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol 168(4):3160–3172. https://doi.org/10.1016/j.ijcard.2013.07.150
Sletten AC, Peterson LR, Schaffer JE (2018) Manifestations and mechanisms of myocardial lipotoxicity in obesity. J Intern Med 284(5):478–491. https://doi.org/10.1111/joim.12728
Roul D, Recchia FA (2015) Metabolic alterations induce oxidative stress in diabetic and failing hearts: different pathways, same outcome. Antioxid Redox Signal 22(17):1502–1514. https://doi.org/10.1089/ars.2015.6311
Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z et al (2004) Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109(18):2191–2196. https://doi.org/10.1161/01.CIR.0000127959.28627.F8
Chen Z, Yu Y, Cai J, Li H (2019) Emerging molecular targets for treatment of nonalcoholic fatty liver disease. Trends Endocrinol Metab 30(12):903–914. https://doi.org/10.1016/j.tem.2019.08.006
Folmes CD, Lopaschuk GD (2007) Role of malonyl-CoA in heart disease and the hypothalamic control of obesity. Cardiovasc Res 73(2):278–287. https://doi.org/10.1016/j.cardiores.2006.10.008
Zhang XJ, Cai J, Li H (2021) Targeting ACC for NASH resolution. Trends Mol Med. https://doi.org/10.1016/j.molmed.2021.11.002
Jian C, Fu J, Cheng X, Shen LJ, Ji YX, Wang X et al (2020) Low-dose sorafenib acts as a mitochondrial uncoupler and ameliorates nonalcoholic steatohepatitis. Cell Metab 31(5):892–908 e11. https://doi.org/10.1016/j.cmet.2020.04.011
Stanley WC, Lopaschuk GD, Hall JL, McCormack JG (1997) Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions Cardiovasc Res 33(2):243–257. https://doi.org/10.1016/s0008-6363(96)00245-3
Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Morris DL, Craig JC (1985) Metabolic fate of extracted glucose in normal human myocardium. J Clin Invest 76(5):1819–1827. https://doi.org/10.1172/JCI112174
Davey KA, Garlick PB, Warley A, Southworth R (2007) Immunogold labeling study of the distribution of GLUT-1 and GLUT-4 in cardiac tissue following stimulation by insulin or ischemia. Am J Physiol Heart Circ Physiol 292(4):H2009–H2019. https://doi.org/10.1152/ajpheart.00663.2006
Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1(7285):785–9. https://doi.org/10.1016/s0140-6736(63)91500-9
Luo P, Wang PX, Li ZZ, Zhang XJ, Jiang X, Gong J et al (2016) Hepatic oncostatin M receptor beta regulates obesity-induced steatosis and insulin resistance. Am J Pathol 186(5):1278–1292. https://doi.org/10.1016/j.ajpath.2015.12.028
Wang XA, Deng S, Jiang D, Zhang R, Zhang S, Zhong J et al (2013) CARD3 deficiency exacerbates diet-induced obesity, hepatosteatosis, and insulin resistance in male mice. Endocrinology 154(2):685–697. https://doi.org/10.1210/en.2012-1911
Yan FJ, Zhang XJ, Wang WX, Ji YX, Wang PX, Yang Y et al (2017) The E3 ligase tripartite motif 8 targets TAK1 to promote insulin resistance and steatohepatitis. Hepatology 65(5):1492–1511. https://doi.org/10.1002/hep.28971
Zhao YC, Zhao GJ, Chen Z, She ZG, Cai J, Li H (2020) Nonalcoholic fatty liver disease: an emerging driver of hypertension. Hypertension 75(2):275–284. https://doi.org/10.1161/HYPERTENSIONAHA.119.13419
Herance JR, Martin-Saladich Q, Velasquez MA, Hernandez C, Aparicio C, Ramirez-Serra C et al (2022) Identification of myocardial insulin resistance by using liver tests: a simple approach for clinical practice. Int J Mol Sci 23(15). https://doi.org/10.3390/ijms23158783
Succurro E, Pedace E, Andreozzi F, Papa A, Vizza P, Fiorentino TV et al (2020) Reduction in global myocardial glucose metabolism in subjects with 1-hour postload hyperglycemia and impaired glucose tolerance. Diabetes Care 43(3):669–676. https://doi.org/10.2337/dc19-1975
Iozzo P, Chareonthaitawee P, Dutka D, Betteridge DJ, Ferrannini E, Camici PG (2002) Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes 51(10):3020–3024. https://doi.org/10.2337/diabetes.51.10.3020
Hu L, Qiu C, Wang X, Xu M, Shao X, Wang Y (2018) The association between diabetes mellitus and reduction in myocardial glucose uptake: a population-based (18)F-FDG PET/CT study. BMC Cardiovasc Disord 18(1):203. https://doi.org/10.1186/s12872-018-0943-9
Taegtmeyer H, Beauloye C, Harmancey R, Hue L (2013) Insulin resistance protects the heart from fuel overload in dysregulated metabolic states. Am J Physiol Heart Circ Physiol 305(12):H1693–H1697. https://doi.org/10.1152/ajpheart.00854.2012
Harmancey R, Wilson CR, Taegtmeyer H (2008) Adaptation and maladaptation of the heart in obesity. Hypertension 52(2):181–187. https://doi.org/10.1161/HYPERTENSIONAHA.108.110031
Hue L, Taegtmeyer H (2009) The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 297(3):E578–E591. https://doi.org/10.1152/ajpendo.00093.2009
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356(24):2457–2471. https://doi.org/10.1056/NEJMoa072761
Peterson LR (2006) Obesity and insulin resistance: effects on cardiac structure, function, and substrate metabolism. Curr Hypertens Rep 8(6):451–456. https://doi.org/10.1007/s11906-006-0022-y
Lopaschuk GD, Folmes CD, Stanley WC (2007) Cardiac energy metabolism in obesity. Circ Res 101(4):335–347. https://doi.org/10.1161/CIRCRESAHA.107.150417
Herrero P, Peterson LR, McGill JB, Matthew S, Lesniak D, Dence C et al (2006) Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol 47(3):598–604. https://doi.org/10.1016/j.jacc.2005.09.030
Mellor KM, Ritchie RH, Delbridge LM (2010) Reactive oxygen species and insulin-resistant cardiomyopathy. Clin Exp Pharmacol Physiol 37(2):222–228. https://doi.org/10.1111/j.1440-1681.2009.05274.x
Witteles RM, Fowler MB (2008) Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol 51(2):93–102. https://doi.org/10.1016/j.jacc.2007.10.021
Grymyr LMD, Nadirpour S, Gerdts E, Nedrebo BG, Hjertaas JJ, Matre K et al (2021) Left ventricular myocardial oxygen demand and subclinical dysfunction in patients with severe obesity referred for bariatric surgery. Nutr Metab Cardiovasc Dis 31(2):666–674. https://doi.org/10.1016/j.numecd.2020.10.009
Hannukainen JC, Lautamaki R, Parkka J, Strandberg M, Saunavaara V, Hurme S et al (2018) Reversibility of myocardial metabolism and remodelling in morbidly obese patients 6 months after bariatric surgery. Diabetes Obes Metab 20(4):963–973. https://doi.org/10.1111/dom.13183
Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W et al (2003) Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med 49(3):417–423. https://doi.org/10.1002/mrm.10372
Wende AR, Symons JD, Abel ED (2012) Mechanisms of lipotoxicity in the cardiovascular system. Curr Hypertens Rep 14(6):517–531. https://doi.org/10.1007/s11906-012-0307-2
Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S et al (2008) Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 52(22):1793–1799. https://doi.org/10.1016/j.jacc.2008.07.062
Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Hooi Ewe S et al (2010) Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 122(24):2538–2544. https://doi.org/10.1161/CIRCULATIONAHA.110.955542
Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K et al (2004) Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 18(14):1692–1700. https://doi.org/10.1096/fj.04-2263com
Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z et al (2016) Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133(21):2038–2049. https://doi.org/10.1161/CIRCULATIONAHA.115.020226
Beyer W, Imlay J, Fridovich I (1991) Superoxide dismutases. Prog Nucleic Acid Res Mol Biol 40:221–253. https://doi.org/10.1016/s0079-6603(08)60843-0
Oshino N, Chance B, Sies H, Bucher T (1973) The role of H 2 O 2 generation in perfused rat liver and the reaction of catalase compound I and hydrogen donors. Arch Biochem Biophys 154(1):117–131. https://doi.org/10.1016/0003-9861(73)90040-4
White CR, Brock TA, Chang LY, Crapo J, Briscoe P, Ku D et al (1994) Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci USA 91(3):1044–1048. https://doi.org/10.1073/pnas.91.3.1044
Zhang L, Wang X, Cueto R, Effi C, Zhang Y, Tan H et al (2019) Biochemical basis and metabolic interplay of redox regulation. Redox Biol 26:101284. https://doi.org/10.1016/j.redox.2019.101284
Campbell EL, Colgan SP (2019) Control and dysregulation of redox signalling in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 16(2):106–120. https://doi.org/10.1038/s41575-018-0079-5
Chen Z, Tian R, She Z, Cai J, Li H (2020) Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med 152:116–141. https://doi.org/10.1016/j.freeradbiomed.2020.02.025
Naviaux RK (2012) Oxidative shielding or oxidative stress? J Pharmacol Exp Ther 342(3):608–618. https://doi.org/10.1124/jpet.112.192120
Figueira TR, Barros MH, Camargo AA, Castilho RF, Ferreira JC, Kowaltowski AJ et al (2013) Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid Redox Signal 18(16):2029–2074. https://doi.org/10.1089/ars.2012.4729
Vercesi AE, Castilho RF, Kowaltowski AJ, de Oliveira HCF, de Souza-Pinto NC, Figueira TR et al (2018) Mitochondrial calcium transport and the redox nature of the calcium-induced membrane permeability transition. Free Radic Biol Med 129:1–24. https://doi.org/10.1016/j.freeradbiomed.2018.08.034
Nabeebaccus A, Zhang M, Shah AM (2011) NADPH oxidases and cardiac remodelling. Heart Fail Rev 16(1):5–12. https://doi.org/10.1007/s10741-010-9186-2
Zhang M, Perino A, Ghigo A, Hirsch E, Shah AM (2013) NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid Redox Signal 18(9):1024–1041. https://doi.org/10.1089/ars.2012.4550
Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M et al (2015) Diabetic cardiovascular disease induced by oxidative stress. Int J Mol Sci 16(10):25234–25263. https://doi.org/10.3390/ijms161025234
Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J (2010) Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106(7):1253–1264. https://doi.org/10.1161/CIRCRESAHA.109.213116
Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J (2010) NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 107(35):15565–15570. https://doi.org/10.1073/pnas.1002178107
Parajuli N, Patel VB, Wang W, Basu R, Oudit GY (2014) Loss of NOX2 (gp91phox) prevents oxidative stress and progression to advanced heart failure. Clin Sci (Lond) 127(5):331–340. https://doi.org/10.1042/CS20130787
Zhao GJ, Zhao CL, Ouyang S, Deng KQ, Zhu L, Montezano AC et al (2020) Ca(2+)-dependent NOX5 (NADPH oxidase 5) exaggerates cardiac hypertrophy through reactive oxygen species production. Hypertension 76(3):827–838. https://doi.org/10.1161/HYPERTENSIONAHA.120.15558
Dhalla NS, Shah AK, Tappia PS (2020) Role of oxidative stress in metabolic and subcellular abnormalities in diabetic cardiomyopathy. Int J Mol Sci 21(7). https://doi.org/10.3390/ijms21072413
Saotome M, Ikoma T, Hasan P, Maekawa Y (2019) Cardiac insulin resistance in heart failure: the role of mitochondrial dynamics. Int J Mol Sci 20(14). https://doi.org/10.3390/ijms20143552
Schonfeld P, Wojtczak L (2008) Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med 45(3):231–241. https://doi.org/10.1016/j.freeradbiomed.2008.04.029
Schonfeld P, Wojtczak L (2007) Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim Biophys Acta 1767(8):1032–1040. https://doi.org/10.1016/j.bbabio.2007.04.005
Cocco T, Di Paola M, Papa S, Lorusso M (1999) Arachidonic acid interaction with the mitochondrial electron transport chain promotes reactive oxygen species generation. Free Radic Biol Med 27(1–2):51–59. https://doi.org/10.1016/s0891-5849(99)00034-9
Loskovich MV, Grivennikova VG, Cecchini G, Vinogradov AD (2005) Inhibitory effect of palmitate on the mitochondrial NADH: ubiquinone oxidoreductase (complex I) as related to the active-de-active enzyme transition. Biochem J 387(Pt 3):677–683. https://doi.org/10.1042/BJ20041703
Schonfeld P, Reiser G (2006) Rotenone-like action of the branched-chain phytanic acid induces oxidative stress in mitochondria. J Biol Chem 281(11):7136–7142. https://doi.org/10.1074/jbc.M513198200
Stillwell W, Jenski LJ, Crump FT, Ehringer W (1997) Effect of docosahexaenoic acid on mouse mitochondrial membrane properties. Lipids 32(5):497–506. https://doi.org/10.1007/s11745-997-0064-6
Schonfeld P, Struy H (1999) Refsum disease diagnostic marker phytanic acid alters the physical state of membrane proteins of liver mitochondria. FEBS Lett 457(2):179–183. https://doi.org/10.1016/s0014-5793(99)01009-1
Gille L, Nohl H (2001) The ubiquinol/bc1 redox couple regulates mitochondrial oxygen radical formation. Arch Biochem Biophys 388(1):34–38. https://doi.org/10.1006/abbi.2000.2257
Skulachev VP (1991) Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett 294(3):158–162. https://doi.org/10.1016/0014-5793(91)80658-p
Korshunov SS, Korkina OV, Ruuge EK, Skulachev VP, Starkov AA (1998) Fatty acids as natural uncouplers preventing generation of O2- and H2O2 by mitochondria in the resting state. FEBS Lett 435(2–3):215–8. https://doi.org/10.1016/s0014-5793(98)01073-4
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3):909–950. https://doi.org/10.1152/physrev.00026.2013
Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47(4):333–343. https://doi.org/10.1016/j.freeradbiomed.2009.05.004
Zhang Y, Bharathi SS, Beck ME, Goetzman ES (2019) The fatty acid oxidation enzyme long-chain acyl-CoA dehydrogenase can be a source of mitochondrial hydrogen peroxide. Redox Biol 26:101253. https://doi.org/10.1016/j.redox.2019.101253
Cardoso AR, Kakimoto PA, Kowaltowski AJ (2013) Diet-sensitive sources of reactive oxygen species in liver mitochondria: role of very long chain acyl-CoA dehydrogenases. PLoS ONE 8(10):e77088. https://doi.org/10.1371/journal.pone.0077088
Montgomery MK, Osborne B, Brown SH, Small L, Mitchell TW, Cooney GJ et al (2013) Contrasting metabolic effects of medium- versus long-chain fatty acids in skeletal muscle. J Lipid Res 54(12):3322–3333. https://doi.org/10.1194/jlr.M040451
Kakimoto PA, Tamaki FK, Cardoso AR, Marana SR, Kowaltowski AJ (2015) H2O2 release from the very long chain acyl-CoA dehydrogenase. Redox Biol 4:375–380. https://doi.org/10.1016/j.redox.2015.02.003
Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED (2005) Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112(17):2686–2695. https://doi.org/10.1161/CIRCULATIONAHA.105.554360
Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O et al (2009) Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 119(9):1272–1283. https://doi.org/10.1161/CIRCULATIONAHA.108.792101
Simoes ICM, Fontes A, Pinton P, Zischka H, Wieckowski MR (2018) Mitochondria in non-alcoholic fatty liver disease. Int J Biochem Cell Biol 95:93–99. https://doi.org/10.1016/j.biocel.2017.12.019
Muftuoglu M, Mori MP, de Souza-Pinto NC (2014) Formation and repair of oxidative damage in the mitochondrial DNA. Mitochondrion 17:164–181. https://doi.org/10.1016/j.mito.2014.03.007
Kawahara H, Fukura M, Tsuchishima M, Takase S (2007) Mutation of mitochondrial DNA in livers from patients with alcoholic hepatitis and nonalcoholic steatohepatitis. Alcohol Clin Exp Res 31(1 Suppl):S54-60. https://doi.org/10.1111/j.1530-0277.2006.00287.x
Marin-Garcia J (2016) Mitochondrial DNA repair: a novel therapeutic target for heart failure. Heart Fail Rev 21(5):475–487. https://doi.org/10.1007/s10741-016-9543-x
Bradley JM, Li Z, Organ CL, Polhemus DJ, Otsuka H, Islam KN et al (2018) A novel mtDNA repair fusion protein attenuates maladaptive remodeling and preserves cardiac function in heart failure. Am J Physiol Heart Circ Physiol 314(2):H311–H321. https://doi.org/10.1152/ajpheart.00515.2017
Cividini F, Scott BT, Dai A, Han W, Suarez J, Diaz-Juarez J et al (2016) O-GlcNAcylation of 8-oxoguanine DNA glycosylase (Ogg1) impairs oxidative mitochondrial DNA lesion repair in diabetic hearts. J Biol Chem 291(51):26515–26528. https://doi.org/10.1074/jbc.M116.754481
Gredilla R, Sanchez-Roman I, Gomez A, Lopez-Torres M, Barja G (2020) Mitochondrial base excision repair positively correlates with longevity in the liver and heart of mammals. Geroscience 42(2):653–665. https://doi.org/10.1007/s11357-020-00158-4
Lee SR, Han J (2017) Mitochondrial mutations in cardiac disorders. Adv Exp Med Biol 982:81–111. https://doi.org/10.1007/978-3-319-55330-6_5
Pohjoismaki JL, Goffart S, Tyynismaa H, Willcox S, Ide T, Kang D et al (2009) Human heart mitochondrial DNA is organized in complex catenated networks containing abundant four-way junctions and replication forks. J Biol Chem 284(32):21446–21457. https://doi.org/10.1074/jbc.M109.016600
Miller FJ, Rosenfeldt FL, Zhang C, Linnane AW, Nagley P (2003) Precise determination of mitochondrial DNA copy number in human skeletal and cardiac muscle by a PCR-based assay: lack of change of copy number with age. Nucleic Acids Res 31(11):e61. https://doi.org/10.1093/nar/gng060
Frahm T, Mohamed SA, Bruse P, Gemund C, Oehmichen M, Meissner C (2005) Lack of age-related increase of mitochondrial DNA amount in brain, skeletal muscle and human heart. Mech Ageing Dev 126(11):1192–1200. https://doi.org/10.1016/j.mad.2005.06.008
Wang J, Wilhelmsson H, Graff C, Li H, Oldfors A, Rustin P et al (1999) Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat Genet 21(1):133–137. https://doi.org/10.1038/5089
Li H, Wang J, Wilhelmsson H, Hansson A, Thoren P, Duffy J et al (2000) Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proc Natl Acad Sci USA 97(7):3467–3472. https://doi.org/10.1073/pnas.97.7.3467
Marin-Garcia J, Goldenthal MJ, Moe GW (2001) Mitochondrial pathology in cardiac failure. Cardiovasc Res 49(1):17–26. https://doi.org/10.1016/s0008-6363(00)00241-8
Elorza AA, Soffia JP (2021) mtDNA heteroplasmy at the core of aging-associated heart failure. An integrative view of OXPHOS and mitochondrial life cycle in cardiac mitochondrial physiology. Front Cell Dev Biol 9:625020. https://doi.org/10.3389/fcell.2021.625020
Li M, Schonberg A, Schaefer M, Schroeder R, Nasidze I, Stoneking M (2010) Detecting heteroplasmy from high-throughput sequencing of complete human mitochondrial DNA genomes. Am J Hum Genet 87(2):237–249. https://doi.org/10.1016/j.ajhg.2010.07.014
Lakshmanan LN, Yee Z, Ng LF, Gunawan R, Halliwell B, Gruber J (2018) Clonal expansion of mitochondrial DNA deletions is a private mechanism of aging in long-lived animals. Aging Cell 17(5):e12814. https://doi.org/10.1111/acel.12814
Tuppen HA, Blakely EL, Turnbull DM, Taylor RW (2010) Mitochondrial DNA mutations and human disease. Biochim Biophys Acta 1797(2):113–128. https://doi.org/10.1016/j.bbabio.2009.09.005
Li H, Slone J, Fei L, Huang T (2019) Mitochondrial DNA variants and common diseases: a mathematical model for the diversity of age-related mtDNA mutations. Cells 8(6). https://doi.org/10.3390/cells8060608
Martinelli I, Tomassoni D, Moruzzi M, Roy P, Cifani C, Amenta F et al (2020) Cardiovascular changes related to metabolic syndrome: evidence in obese zucker rats. Int J Mol Sci 21(6). https://doi.org/10.3390/ijms21062035
Li J, Romestaing C, Han X, Li Y, Hao X, Wu Y et al (2010) Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab 12(2):154–165. https://doi.org/10.1016/j.cmet.2010.07.003
Tappia PS, Adameova A, Dhalla NS (2018) Attenuation of diabetes-induced cardiac and subcellular defects by sulphur-containing amino acids. Curr Med Chem 25(3):336–345. https://doi.org/10.2174/0929867324666170705115207
Wilson AJ, Gill EK, Abudalo RA, Edgar KS, Watson CJ, Grieve DJ (2018) Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart 104(4):293–299. https://doi.org/10.1136/heartjnl-2017-311448
D’Oria R, Schipani R, Leonardini A, Natalicchio A, Perrini S, Cignarelli A et al (2020) The role of oxidative stress in cardiac disease: from physiological response to injury factor. Oxid Med Cell Longev 2020:5732956. https://doi.org/10.1155/2020/5732956
Quijano C, Trujillo M, Castro L, Trostchansky A (2016) Interplay between oxidant species and energy metabolism. Redox Biol 8:28–42. https://doi.org/10.1016/j.redox.2015.11.010
Zhu LH, Wang A, Luo P, Wang X, Jiang DS, Deng W et al (2014) Mindin/Spondin 2 inhibits hepatic steatosis, insulin resistance, and obesity via interaction with peroxisome proliferator-activated receptor alpha in mice. J Hepatol 60(5):1046–1054. https://doi.org/10.1016/j.jhep.2014.01.011
Tong J, Han CJ, Zhang JZ, He WZ, Zhao GJ, Cheng X et al (2019) Hepatic interferon regulatory factor 6 alleviates liver steatosis and metabolic disorder by transcriptionally suppressing peroxisome proliferator-activated receptor gamma in mice. Hepatology 69(6):2471–2488. https://doi.org/10.1002/hep.30559
Li S, Yang B, Du Y, Lin Y, Liu J, Huang S et al (2018) Targeting PPARalpha for the treatment and understanding of cardiovascular diseases. Cell Physiol Biochem 51(6):2760–2775. https://doi.org/10.1159/000495969
Li JL, Wang QY, Luan HY, Kang ZC, Wang CB (2012) Effects of L-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha. J Biomed Sci 19:32. https://doi.org/10.1186/1423-0127-19-32
Blanquicett C, Kang BY, Ritzenthaler JD, Jones DP, Hart CM (2010) Oxidative stress modulates PPAR gamma in vascular endothelial cells. Free Radic Biol Med 48(12):1618–1625. https://doi.org/10.1016/j.freeradbiomed.2010.03.007
Pizzimenti S, Laurora S, Briatore F, Ferretti C, Dianzani MU, Barrera G (2002) Synergistic effect of 4-hydroxynonenal and PPAR ligands in controlling human leukemic cell growth and differentiation. Free Radic Biol Med 32(3):233–245. https://doi.org/10.1016/s0891-5849(01)00798-5
Marino A, Hausenloy DJ, Andreadou I, Horman S, Bertrand L, Beauloye C (2021) AMP-activated protein kinase: a remarkable contributor to preserve a healthy heart against ROS injury. Free Radic Biol Med 166:238–254. https://doi.org/10.1016/j.freeradbiomed.2021.02.047
Cardaci S, Filomeni G, Ciriolo MR (2012) Redox implications of AMPK-mediated signal transduction beyond energetic clues. J Cell Sci 125(Pt 9):2115–2125. https://doi.org/10.1242/jcs.095216
Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E (2010) Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem 285(43):33154–33164. https://doi.org/10.1074/jbc.M110.143685
Ritchie RH, Quinn JM, Cao AH, Drummond GR, Kaye DM, Favaloro JM et al (2007) The antioxidant tempol inhibits cardiac hypertrophy in the insulin-resistant GLUT4-deficient mouse in vivo. J Mol Cell Cardiol 42(6):1119–1128. https://doi.org/10.1016/j.yjmcc.2007.03.900
Fang CX, Dong F, Ren BH, Epstein PN, Ren J (2005) Metallothionein alleviates cardiac contractile dysfunction induced by insulin resistance: role of Akt phosphorylation, PTB1B. PPARgamma and c-Jun Diabetologia 48(11):2412–2421. https://doi.org/10.1007/s00125-005-1940-y
Turdi S, Li Q, Lopez FL, Ren J (2007) Catalase alleviates cardiomyocyte dysfunction in diabetes: role of Akt, Forkhead transcriptional factor and silent information regulator 2. Life Sci 81(11):895–905. https://doi.org/10.1016/j.lfs.2007.07.029
Di Meo S, Iossa S, Venditti P (2017) Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J Endocrinol 233(1):R15–R42. https://doi.org/10.1530/JOE-16-0598
Rindler PM, Crewe CL, Fernandes J, Kinter M, Szweda LI (2013) Redox regulation of insulin sensitivity due to enhanced fatty acid utilization in the mitochondria. Am J Physiol Heart Circ Physiol 305(5):H634–H643. https://doi.org/10.1152/ajpheart.00799.2012
Boden MJ, Brandon AE, Tid-Ang JD, Preston E, Wilks D, Stuart E et al (2012) Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Am J Physiol Endocrinol Metab 303(6):E798-805. https://doi.org/10.1152/ajpendo.00577.2011
Lark DS, Kang L, Lustig ME, Bonner JS, James FD, Neufer PD et al (2015) Enhanced mitochondrial superoxide scavenging does not improve muscle insulin action in the high fat-fed mouse. PLoS ONE 10(5):e0126732. https://doi.org/10.1371/journal.pone.0126732
Forman HJ, Maiorino M, Ursini F (2010) Signaling functions of reactive oxygen species. Biochemistry 49(5):835–842. https://doi.org/10.1021/bi9020378
Iwakami S, Misu H, Takeda T, Sugimori M, Matsugo S, Kaneko S et al (2011) Concentration-dependent dual effects of hydrogen peroxide on insulin signal transduction in H4IIEC hepatocytes. PLoS ONE 6(11):e27401. https://doi.org/10.1371/journal.pone.0027401
Cai J, Xu M, Zhang X, Li H (2019) Innate immune signaling in nonalcoholic fatty liver disease and cardiovascular diseases. Annu Rev Pathol 14:153–184. https://doi.org/10.1146/annurev-pathmechdis-012418-013003
Deng KQ, Zhao GN, Wang Z, Fang J, Jiang Z, Gong J et al (2018) Targeting transmembrane BAX inhibitor motif containing 1 alleviates pathological cardiac hypertrophy. Circulation 137(14):1486–1504. https://doi.org/10.1161/CIRCULATIONAHA.117.031659
Ji YX, Zhang P, Zhang XJ, Zhao YC, Deng KQ, Jiang X et al (2016) The ubiquitin E3 ligase TRAF6 exacerbates pathological cardiac hypertrophy via TAK1-dependent signalling. Nat Commun 7:11267. https://doi.org/10.1038/ncomms11267
Chen L, Huang J, Ji YX, Mei F, Wang PX, Deng KQ et al (2017) Tripartite motif 8 contributes to pathological cardiac hypertrophy through enhancing transforming growth factor beta-activated kinase 1-dependent signaling pathways. Hypertension 69(2):249–258. https://doi.org/10.1161/HYPERTENSIONAHA.116.07741
Zhang Y, Li H (2017) Reprogramming interferon regulatory factor signaling in cardiometabolic diseases. Physiology (Bethesda) 32(3):210–223. https://doi.org/10.1152/physiol.00038.2016
Zhang XJ, Zhang P, Li H (2015) Interferon regulatory factor signalings in cardiometabolic diseases. Hypertension 66(2):222–247. https://doi.org/10.1161/HYPERTENSIONAHA.115.04898
Zhang XJ, Liu X, Hu M, Zhao GJ, Sun D, Cheng X et al (2021) Pharmacological inhibition of arachidonate 12-lipoxygenase ameliorates myocardial ischemia-reperfusion injury in multiple species. Cell Metab 33(10):2059–75 e10. https://doi.org/10.1016/j.cmet.2021.08.014
Deng KQ, Wang A, Ji YX, Zhang XJ, Fang J, Zhang Y et al (2016) Suppressor of IKKvarepsilon is an essential negative regulator of pathological cardiac hypertrophy. Nat Commun 7:11432. https://doi.org/10.1038/ncomms11432
Xu M, Liu PP, Li H (2019) Innate immune signaling and its role in metabolic and cardiovascular diseases. Physiol Rev 99(1):893–948. https://doi.org/10.1152/physrev.00065.2017
Zhang Y, Zhang XJ, Wang PX, Zhang P, Li H (2017) Reprogramming innate immune signaling in cardiometabolic disease. Hypertension 69(5):747–760. https://doi.org/10.1161/HYPERTENSIONAHA.116.08192
Zhang Y, Zhang XJ, Li H (2017) Targeting interferon regulatory factor for cardiometabolic diseases: opportunities and challenges. Curr Drug Targets 18(15):1754–1778. https://doi.org/10.2174/1389450116666150804110412
Wang W, Zhang Y, Yang L, Li H (2017) The innate immune signaling in cancer and cardiometabolic diseases: friends or foes? Cancer Lett 387:46–60. https://doi.org/10.1016/j.canlet.2016.06.004
Al-Khafaji AB, Tohme S, Yazdani HO, Miller D, Huang H, Tsung A (2016) Superoxide induces neutrophil extracellular trap formation in a TLR-4 and NOX-dependent mechanism. Mol Med 22:621–631. https://doi.org/10.2119/molmed.2016.00054
Frantz S, Kelly RA, Bourcier T (2001) Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem 276(7):5197–5203. https://doi.org/10.1074/jbc.M009160200
Doridot L, Jeljeli M, Chene C, Batteux F (2019) Implication of oxidative stress in the pathogenesis of systemic sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol 25:101122. https://doi.org/10.1016/j.redox.2019.101122
Abais JM, Xia M, Li G, Gehr TW, Boini KM, Li PL (2014) Contribution of endogenously produced reactive oxygen species to the activation of podocyte NLRP3 inflammasomes in hyperhomocysteinemia. Free Radic Biol Med 67:211–220. https://doi.org/10.1016/j.freeradbiomed.2013.10.009
Bae JY, Park HH (2011) Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J Biol Chem 286(45):39528–39536. https://doi.org/10.1074/jbc.M111.278812
Win S, Than TA, Fernandez-Checa JC, Kaplowitz N (2014) JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis 5:e989. https://doi.org/10.1038/cddis.2013.522
Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S et al (2003) Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA 100(26):15883–15888. https://doi.org/10.1073/pnas.2136717100
Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J et al (2005) Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem 280(44):37033–37040. https://doi.org/10.1074/jbc.M506771200
Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y et al (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17(9):2596–2606. https://doi.org/10.1093/emboj/17.9.2596
Sekine Y, Hatanaka R, Watanabe T, Sono N, Iemura S, Natsume T et al (2012) The Kelch repeat protein KLHDC10 regulates oxidative stress-induced ASK1 activation by suppressing PP5. Mol Cell 48(5):692–704. https://doi.org/10.1016/j.molcel.2012.09.018
Yadav UC, Ramana KV (2013) Regulation of NF-kappaB-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxid Med Cell Longev 2013:690545. https://doi.org/10.1155/2013/690545
Furnkranz A, Leitinger N (2004) Regulation of inflammatory responses by oxidized phospholipids: structure-function relationships. Curr Pharm Des 10(8):915–921. https://doi.org/10.2174/1381612043452929
Sanchez-Trujillo L, Vazquez-Garza E, Castillo EC, Garcia-Rivas G, Torre-Amione G (2017) Role of adaptive immunity in the development and progression of heart failure: new evidence. Arch Med Res 48(1):1–11. https://doi.org/10.1016/j.arcmed.2016.12.008
Desdin-Mico G, Soto-Heredero G, Aranda JF, Oller J, Carrasco E, Gabande-Rodriguez E et al (2020) T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368(6497):1371–1376. https://doi.org/10.1126/science.aax0860
Sun L, Wang X, Saredy J, Yuan Z, Yang X, Wang H (2020) Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol 37:101759. https://doi.org/10.1016/j.redox.2020.101759
Kaminski MM, Sauer SW, Klemke CD, Suss D, Okun JG, Krammer PH et al (2010) Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J Immunol 184(9):4827–4841. https://doi.org/10.4049/jimmunol.0901662
Chen X, Song M, Zhang B, Zhang Y (2016) Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxid Med Cell Longev 2016:1580967. https://doi.org/10.1155/2016/1580967
Frossi B, De Carli M, Piemonte M, Pucillo C (2008) Oxidative microenvironment exerts an opposite regulatory effect on cytokine production by Th1 and Th2 cells. Mol Immunol 45(1):58–64. https://doi.org/10.1016/j.molimm.2007.05.008
Kraaij MD, Savage ND, van der Kooij SW, Koekkoek K, Wang J, van den Berg JM et al (2010) Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc Natl Acad Sci USA 107(41):17686–17691. https://doi.org/10.1073/pnas.1012016107
Wheeler ML, Defranco AL (2012) Prolonged production of reactive oxygen species in response to B cell receptor stimulation promotes B cell activation and proliferation. J Immunol 189(9):4405–4416. https://doi.org/10.4049/jimmunol.1201433
Carvajal K, Balderas-Villalobos J, Bello-Sanchez MD, Phillips-Farfan B, Molina-Munoz T, Aldana-Quintero H et al (2014) Ca(2+) mishandling and cardiac dysfunction in obesity and insulin resistance: role of oxidative stress. Cell Calcium 56(5):408–415. https://doi.org/10.1016/j.ceca.2014.08.003
van der Pol A, van Gilst WH, Voors AA, van der Meer P (2019) Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail 21(4):425–435. https://doi.org/10.1002/ejhf.1320
Lancel S, Qin F, Lennon SL, Zhang J, Tong X, Mazzini MJ et al (2010) Oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Galphaq-overexpressing mice. Circ Res 107(2):228–232. https://doi.org/10.1161/CIRCRESAHA.110.217570
Li SY, Yang X, Ceylan-Isik AF, Du M, Sreejayan N, Ren J (2006) Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia 49(6):1434–1446. https://doi.org/10.1007/s00125-006-0229-0
Dong F, Fang CX, Yang X, Zhang X, Lopez FL, Ren J (2006) Cardiac overexpression of catalase rescues cardiac contractile dysfunction induced by insulin resistance: Role of oxidative stress, protein carbonyl formation and insulin sensitivity. Diabetologia 49(6):1421–1433. https://doi.org/10.1007/s00125-006-0230-7
Balderas-Villalobos J, Molina-Munoz T, Mailloux-Salinas P, Bravo G, Carvajal K, Gomez-Viquez NL (2013) Oxidative stress in cardiomyocytes contributes to decreased SERCA2a activity in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol 305(9):H1344–H1353. https://doi.org/10.1152/ajpheart.00211.2013
Gomez-Viquez NL, Balderas-Villalobos J, Bello-Sanchez MD, Mayorga-Luna M, Mailloux-Salinas P, Garcia-Castaneda M et al (2021) Oxidative stress in early metabolic syndrome impairs cardiac RyR2 and SERCA2a activity and modifies the interplay of these proteins during Ca(2+) waves. Arch Physiol Biochem 1–13. https://doi.org/10.1080/13813455.2021.1895224
Cesselli D, Jakoniuk I, Barlucchi L, Beltrami AP, Hintze TH, Nadal-Ginard B et al (2001) Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ Res 89(3):279–286. https://doi.org/10.1161/hh1501.094115
Barlaka E, Gorbe A, Gaspar R, Paloczi J, Ferdinandy P, Lazou A (2015) Activation of PPARbeta/delta protects cardiac myocytes from oxidative stress-induced apoptosis by suppressing generation of reactive oxygen/nitrogen species and expression of matrix metalloproteinases. Pharmacol Res 95–96:102–110. https://doi.org/10.1016/j.phrs.2015.03.008
Laviola L, Leonardini A, Melchiorre M, Orlando MR, Peschechera A, Bortone A et al (2012) Glucagon-like peptide-1 counteracts oxidative stress-dependent apoptosis of human cardiac progenitor cells by inhibiting the activation of the c-Jun N-terminal protein kinase signaling pathway. Endocrinology 153(12):5770–5781. https://doi.org/10.1210/en.2012-1461
Leonardini A, D’Oria R, Incalza MA, Caccioppoli C, Andrulli Buccheri V, Cignarelli A et al (2017) GLP-1 receptor activation inhibits palmitate-induced apoptosis via ceramide in human cardiac progenitor cells. J Clin Endocrinol Metab 102(11):4136–4147. https://doi.org/10.1210/jc.2017-00970
Rana S, Datta K, Reddy TL, Chatterjee E, Sen P, Pal-Bhadra M et al (2015) A spatio-temporal cardiomyocyte targeted vector system for efficient delivery of therapeutic payloads to regress cardiac hypertrophy abating bystander effect. J Control Release 200:167–178. https://doi.org/10.1016/j.jconrel.2015.01.008
Zhao X, Luo W, Hu J, Zuo L, Wang J, Hu R et al (2018) Cardiomyocyte-targeted and 17beta-estradiol-loaded acoustic nanoprobes as a theranostic platform for cardiac hypertrophy. J Nanobiotechnology 16(1):36. https://doi.org/10.1186/s12951-018-0360-3
Ramachandra CJA, Chua J, Cong S, Kp MMJ, Shim W, Wu JC et al (2021) Human-induced pluripotent stem cells for modelling metabolic perturbations and impaired bioenergetics underlying cardiomyopathies. Cardiovasc Res 117(3):694–711. https://doi.org/10.1093/cvr/cvaa125
Caudal A, Ren L, Tu C, Wu JC (2022) Human induced pluripotent stem cells for studying mitochondrial diseases in the heart. FEBS Lett 596(14):1735–1745. https://doi.org/10.1002/1873-3468.14444
Vuckovic S, Dinani R, Nollet EE, Kuster DWD, Buikema JW, Houtkooper RH et al (2022) Characterization of cardiac metabolism in iPSC-derived cardiomyocytes: lessons from maturation and disease modeling. Stem Cell Res Ther 13(1):332. https://doi.org/10.1186/s13287-022-03021-9
Venkatesh S, Baljinnyam E, Tong M, Kashihara T, Yan L, Liu T et al (2021) Proteomic analysis of mitochondrial biogenesis in cardiomyocytes differentiated from human induced pluripotent stem cells. Am J Physiol Regul Integr Comp Physiol 320(4):R547–R562. https://doi.org/10.1152/ajpregu.00207.2020
Hallas T, Eisen B, Shemer Y, Ben Jehuda R, Mekies LN, Naor S et al (2018) Investigating the cardiac pathology of SCO2-mediated hypertrophic cardiomyopathy using patients induced pluripotent stem cell-derived cardiomyocytes. J Cell Mol Med 22(2):913–925. https://doi.org/10.1111/jcmm.13392
Zhan Y, Sun X, Li B, Cai H, Xu C, Liang Q et al (2018) Establishment of a PRKAG2 cardiac syndrome disease model and mechanism study using human induced pluripotent stem cells. J Mol Cell Cardiol 117:49–61. https://doi.org/10.1016/j.yjmcc.2018.02.007
Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A et al (2014) Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 20(6):616–623. https://doi.org/10.1038/nm.3545
Wu JC, Garg P, Yoshida Y, Yamanaka S, Gepstein L, Hulot JS et al (2019) Towards precision medicine with human iPSCs for cardiac channelopathies. Circ Res 125(6):653–658. https://doi.org/10.1161/CIRCRESAHA.119.315209
Funding
This work was supported by grants from the Hubei Province Innovation Platform Construction Project (20204201117303072238), Wuhan Science and Technology Planning Project (2020021105012439), the National Science Foundation of China (82000386, 81870171), and the Excellent Doctoral Program of Zhongnan Hospital of Wuhan University (ZNYB2019001).
Author information
Authors and Affiliations
Contributions
All authors contributed toward this work. Conceptualization: Huo-Ping Li, Zhibing Lu, and Hongliang Li; literature search and analysis: Ze Chen, Zhao-Xia Jin, Ruyan Li, Ke-Qiong Deng, Yan-Xiao Ji, and Fang Lei; writing of the first draft of the manuscript: Ze Chen and Zhao-Xia Jin; review and editing: Jingjing Cai, Ruyan Li, Huo-Ping Li, Zhibing Lu, and Hongliang Li. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All the authors give their consent for participation.
Consent for publication
All the authors give their consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Z., Jin, ZX., Cai, J. et al. Energy substrate metabolism and oxidative stress in metabolic cardiomyopathy. J Mol Med 100, 1721–1739 (2022). https://doi.org/10.1007/s00109-022-02269-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02269-1