Abstract
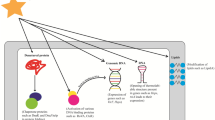
Temperature is among the most important of the parameters that free-living microbes monitor. Microbial physiology needs to be readjusted in response to sudden temperature changes. When the ambient temperature rises or drops to potentially harmful levels, cells mount protective stress responses—so-called heat or cold shock responses, respectively. Pathogenic microorganisms often respond to a temperature of around 37°C by inducing virulence gene expression. There are two main ways in which temperature can be measured. Often, the consequences of a sudden temperature shift are detected. Such indirect signals are known to be the accumulation of denatured proteins (heat shock) or stalled ribosomes (cold shock). However, this article focuses solely on direct thermosensors. Since the conformation of virtually every biomolecule is susceptible to temperature changes, primary sensors include DNA, RNA, proteins and lipids.




Similar content being viewed by others
References
Guisbert E, Yura T, Rhodius VA, Gross CA (2008) Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiol Mol Biol Rev 72:545–554
Zhao K, Liu M, Burgess RR (2005) The global transcriptional response of Escherichia coli to induced σ32 protein involves σ32 regulon activation followed by inactivation and degradation of σ32 in vivo. J Biol Chem 280:17758–17768
Nonaka G, Blankschien M, Herman C, Gross CA, Rhodius VA (2006) Regulon and promoter analysis of the E. coli heat-shock factor, σ32, reveals a multifaceted cellular response to heat stress. Genes Dev 20:1776–1789
Wade JT, Roa DC, Grainger DC, Hurd D, Busby SJ, Struhl K, Nudler E (2006) Extensive functional overlap between sigma factors in Escherichia coli. Nat Struct Mol Biol 13:806–814
Meibom KL, Dubail I, Dupuis M, Barel M, Lenco J, Stulik J, Golovliov I, Sjostedt A, Charbit A (2008) The heat-shock protein ClpB of Francisella tularensis is involved in stress tolerance and is required for multiplication in target organs of infected mice. Mol Microbiol 67:1384–1401
Schumann W (2007) Thermosensors in eubacteria: role and evolution. J Biosci 32:549–557
Huston WM, Theodoropoulos C, Mathews SA, Timms P (2008) Chlamydia trachomatis responds to heat shock, penicillin induced persistence, and IFN-gamma persistence by altering levels of the extracytoplasmic stress response protease HtrA. BMC Microbiol 8:190
Konkel ME, Tilly K (2000) Temperature-regulated expression of bacterial virulence genes. Microbes Infect 2:157–166
Jin S, Song YN, Deng WY, Gordon MP, Nester EW (1993) The regulatory VirA protein of Agrobacterium tumefaciens does not function at elevated temperatures. J Bacteriol 175:6830–6835
Banta LM, Bohne J, Lovejoy SD, Dostal K (1998) Stability of the Agrobacterium tumefaciens VirB10 protein is modulated by growth temperature and periplasmic osmoadaption. J Bacteriol 180:6597–6606
Lai EM, Kado CI (1998) Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J Bacteriol 180:2711–2717
Wei ZM, Sneath BJ, Beer SV (1992) Expression of Erwinia amylovora hrp genes in response to environmental stimuli. J Bacteriol 174:1875–1882
van Dijk K, Fouts DE, Rehm AH, Hill AR, Collmer A, Alfano JR (1999) The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (type III) protein secretion system in a temperature- and pH-sensitive manner. J Bacteriol 181:4790–4797
Lanham PG, Mcllravey KI, Perombelon MCM (1991) Production of cell wall dissolving enzymes by Erwinia carotovora subsp. atroseptica in vitro at 27°C and 30°C. J Appl Microbiol 70:20–24
Smirnova A, Li H, Weingart H, Aufhammer S, Burse A, Finis K, Schenk A, Ullrich MS (2001) Thermoregulated expression of virulence factors in plant-associated bacteria. Arch Microbiol 176:393–399
Ermolenko DN, Makhatadze GI (2002) Bacterial cold-shock proteins. Cell Mol Life Sci 59:1902–1913
Horn G, Hofweber R, Kremer W, Kalbitzer HR (2007) Structure and function of bacterial cold shock proteins. Cell Mol Life Sci 64:1457–1470
El-Sharoud WM, Graumann PL (2007) Cold shock proteins aid coupling of transcription and translation in bacteria. Sci Prog 90:15–27
Narberhaus F (1999) Negative regulation of bacterial heat shock genes. Mol Microbiol 31:1–8
Crick F (1970) Central dogma of molecular biology. Nature 227:561–563
Lopez-Garcia P, Forterre P (2000) DNA topology and the thermal stress response, a tale from mesophiles and hyperthermophiles. Bioessays 22:738–746
Kataoka K, Mizushima T, Ogata Y, Miki T, Sekimizu K (1996) Heat shock-induced DNA relaxation in vitro by DNA gyrase of Escherichia coli in the presence of ATP. J Biol Chem 271:24806–24810
Mizushima T, Kataoka K, Ogata Y, Inoue R, Sekimizu K (1997) Increase in negative supercoiling of plasmid DNA in Escherichia coli exposed to cold shock. Mol Microbiol 23:381–386
Lopez-Garcia P, Forterre P (1997) DNA topology in hyperthermophilic archaea: reference states and their variation with growth phase, growth temperature, and temperature stresses. Mol Microbiol 23:1267–1279
Pruss GJ, Drlica K (1989) DNA supercoiling and prokaryotic transcription. Cell 56:521–523
Dorman CJ (1996) Flexible response: DNA supercoiling, transcription and bacterial adaptation to environmental stress. Trends Microbiol 4:214–216
Dorman CJ, Corcoran CP (2008) Bacterial DNA topology and infectious disease. Nucleic Acids Res 37:672–678
Bell SD, Jaxel C, Nadal M, Kosa PF, Jackson SP (1998) Temperature, template topology, and factor requirements of archaeal transcription. Proc Natl Acad Sci USA 95:15218–15222
Katayama S, Matsushita O, Tamai E, Miyata S, Okabe A (2001) Phased A-tracts bind to the alpha subunit of RNA polymerase with increased affinity at low temperature. FEBS Lett 509:235–238
Mizuno T (1987) Random cloning of bent DNA segments from Escherichia coli chromosome and primary characterization of their structures. Nucleic Acids Res 15:6827–6841
Nickerson CA, Achberger EC (1995) Role of curved DNA in binding of Escherichia coli RNA polymerase to promoters. J Bacteriol 177:5756–5761
Katayama S, Matsushita O, Jung CM, Minami J, Okabe A (1999) Promoter upstream bent DNA activates the transcription of the Clostridium perfringens phospholipase C gene in a low temperature-dependent manner. EMBO J 18:3442–3450
Los DA (2004) The effect of low-temperature-induced DNA supercoiling on the expression of the desaturase genes in Synechocystis. Cell Mol Biol (Noisy-le-Grand) 50:605–612
Prosseda G, Falconi M, Giangrossi M, Gualerzi CO, Micheli G, Colonna B (2004) The virF promoter in Shigella: more than just a curved DNA stretch. Mol Microbiol 51:523–537
Tupper AE, Owen-Hughes TA, Ussery DW, Santos DS, Ferguson DJ, Sidebotham JM, Hinton JC, Higgins CF (1994) The chromatin-associated protein H-NS alters DNA topology in vitro. EMBO J 13:258–268
Falconi M, Colonna B, Prosseda G, Micheli G, Gualerzi CO (1998) Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. EMBO J 17:7033–7043
Ono S, Goldberg MD, Olsson T, Esposito D, Hinton JC, Ladbury JE (2005) H-NS is a part of a thermally controlled mechanism for bacterial gene regulation. Biochem J 391:203–213
Atlung T, Ingmer H (1997) H-NS: a modulator of environmentally regulated gene expression. Mol Microbiol 24:7–17
Colonna B, Casalino M, Fradiani PA, Zagaglia C, Naitza S, Leoni L, Prosseda G, Coppo A, Ghelardini P, Nicoletti M (1995) H-NS regulation of virulence gene expression in enteroinvasive Escherichia coli harboring the virulence plasmid integrated into the host chromosome. J Bacteriol 177:4703–4712
Rohde JR, Luan XS, Rohde H, Fox JM, Minnich SA (1999) The Yersinia enterocolitica pYV virulence plasmid contains multiple intrinsic DNA bends which melt at 37°C. J Bacteriol 181:4198–4204
Madrid C, Nieto JM, Paytubi S, Falconi M, Gualerzi CO, Juarez A (2002) Temperature- and H-NS-dependent regulation of a plasmid-encoded virulence operon expressing Escherichia coli hemolysin. J Bacteriol 184:5058–5066
Dorman CJ, Porter ME (1998) The Shigella virulence gene regulatory cascade: a paradigm of bacterial gene control mechanisms. Mol Microbiol 29:677–684
Tobe T, Yoshikawa M, Mizuno T, Sasakawa C (1993) Transcriptional control of the invasion regulatory gene virB of Shigella flexneri: activation by virF and repression by H-NS. J Bacteriol 175:6142–6149
White-Ziegler CA, Davis TR (2009) Genome-wide identification of H-NS-controlled, temperature-regulated genes in Escherichia coli K-12. J Bacteriol 191:1106–1110
Goransson M, Sonden B, Nilsson P, Dagberg B, Forsman K, Emanuelsson K, Uhlin BE (1990) Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature 344:682–685
White-Ziegler CA, Angus Hill ML, Braaten BA, van der Woude MW, Low DA (1998) Thermoregulation of Escherichia coli pap transcription: H-NS is a temperature-dependent DNA methylation blocking factor. Mol Microbiol 28:1121–1137
Duong N, Osborne S, Bustamante VH, Tomljenovic AM, Puente JL, Coombes BK (2007) Thermosensing coordinates a cis-regulatory module for transcriptional activation of the intracellular virulence system in Salmonella enterica serovar Typhimurium. J Biol Chem 282:34077–34084
Mitobe J, Morita-Ishihara T, Ishihama A, Watanabe H (2008) Involvement of RNA-binding protein Hfq in the post-transcriptional regulation of invE gene expression in Shigella sonnei. J Biol Chem 283:5738–5747
Storz G (1999) An RNA thermometer. Genes Dev 13:633–636
Narberhaus F, Waldminghaus T, Chowdhury S (2006) RNA thermometers. FEMS Microbiol Rev 30:3–16
Morita MT, Tanaka Y, Kodama TS, Kyogoku Y, Yanagi H, Yura T (1999) Translational induction of heat shock transcription factor σ32: evidence for a built-in RNA thermosensor. Genes Dev 13:655–665
Narberhaus F, Käser R, Nocker A, Hennecke H (1998) A novel DNA element that controls bacterial heat shock gene expression. Mol Microbiol 28:315–323
Nocker A, Krstulovic NP, Perret X, Narberhaus F (2001) ROSE elements occur in disparate rhizobia and are functionally interchangeable between species. Arch Microbiol 176:44–51
Waldminghaus T, Fippinger A, Alfsmann J, Narberhaus F (2005) RNA thermometers are common in alpha- and gamma-proteobacteria. Biol Chem 386:1279–1286
Nocker A, Hausherr T, Balsiger S, Krstulovic NP, Hennecke H, Narberhaus F (2001) A mRNA-based thermosensor controls expression of rhizobial heat shock genes. Nucleic Acids Res 29:4800–4807
Chowdhury S, Ragaz C, Kreuger E, Narberhaus F (2003) Temperature-controlled structural alterations of an RNA thermometer. J Biol Chem 278:47915–47921
Chowdhury S, Maris C, Allain FHT, Narberhaus F (2006) Molecular basis for temperature sensing by an RNA thermometer. EMBO J 25:2487–2497
Waldminghaus T, Heidrich N, Brantl S, Narberhaus F (2007) FourU: a novel type of RNA thermometer in Salmonella. Mol Microbiol 65:413–424
Hoe NP, Goguen JD (1993) Temperature sensing in Yersinia pestis: translation of the LcrF activator protein is thermally regulated. J Bacteriol 175:7901–7909
Johansson J, Mandin P, Renzoni A, Chiaruttini C, Springer M, Cossart P (2002) An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell 110:551–561
Waldminghaus T, Gaubig LC, Narberhaus F (2007) Genome-wide bioinformatic prediction and experimental evaluation of potential RNA thermometers. Mol Genet Genomics 278:555–564
Waldminghaus T, Kortmann J, Gesing S, Narberhaus F (2008) Generation of synthetic RNA-based thermosensors. Biol Chem 389:1319–1326
Wieland M, Hartig JS (2007) RNA quadruplex-based modulation of gene expression. Chem Biol 14:757–763
Neupert J, Karcher D, Bock R (2008) Design of simple synthetic RNA thermometers for temperature-controlled gene expression in Escherichia coli. Nucleic Acids Res 36:e124
Altuvia S, Kornitzer D, Teff D, Oppenheim AB (1989) Alternative mRNA structures of the cIII gene of bacteriophage lambda determine the rate of its translation initiation. J Mol Biol 210:265–280
Yamanaka K, Mitta M, Inouye M (1999) Mutation analysis of the 5′ untranslated region of the cold shock cspA mRNA of Escherichia coli. J Bacteriol 181:6284–6291
Fang L, Jiang W, Bae W, Inouye M (1997) Promoter-independent cold-shock induction of cspA and its derepression at 37°C by mRNA stabilization. Mol Microbiol 23:355–364
Uppal S, Akkipeddi VS, Jawali N (2008) Posttranscriptional regulation of cspE in Escherichia coli: involvement of the short 5′-untranslated region. FEMS Microbiol Lett 279:83–91
Lybecker MC, Samuels DS (2007) Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol Microbiol 64:1075–1089
Sledjeski DD, Gupta A, Gottesman S (1996) The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J 15:3993–4000
Repoila F, Gottesman S (2003) Temperature sensing by the dsrA promoter. J Bacteriol 185:6609–6614
Morimoto RI (1993) Cells in stress: transcriptional activation of heat shock genes. Science 259:1409–1410
Servant P, Grandvalet C, Mazodier P (2000) The RheA repressor is the thermosensor of the HSP18 heat shock response in Streptomyces albus. Proc Natl Acad Sci USA 97:3538–3543
Servant P, Mazodier P (2001) Negative regulation of the heat shock response in Streptomyces. Arch Microbiol 176:237–242
Hurme R, Berndt KD, Normark SJ, Rhen M (1997) A proteinaceous gene regulatory thermometer in Salmonella. Cell 90:55–64
Gal-Mor O, Valdez Y, Finlay BB (2006) The temperature-sensing protein TlpA is repressed by PhoP and dispensable for virulence of Salmonella enterica serovar Typhimurium in mice. Microbes Infect 8:2154–2162
Naik RR, Kirkpatrick SM, Stone MO (2001) The thermostability of an alpha-helical coiled-coil protein and its potential use in sensor applications. Biosens Bioelectron 16:1051–1057
Liu W, Vierke G, Wenke AK, Thomm M, Ladenstein R (2007) Crystal structure of the archaeal heat shock regulator from Pyrococcus furiosus: a molecular chimera representing eukaryal and bacterial features. J Mol Biol 369:474–488
Maresca B, Kobayashi GS (1989) Dimorphism in Histoplasma capsulatum: a model for the study of cell differentiation in pathogenic fungi. Microbiol Rev 53:186–209
Nguyen VQ, Sil A (2008) Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc Natl Acad Sci USA 105:4880–4885
Nemecek JC, Wuthrich M, Klein BS (2006) Global control of dimorphism and virulence in fungi. Science 312:583–588
Beier D, Gross R (2006) Regulation of bacterial virulence by two-component systems. Curr Opin Microbiol 9:143–152
Calva E, Oropeza R (2006) Two-component signal transduction systems, environmental signals, and virulence. Microb Ecol 51:166–176
Stock AM, Robinson VL, Goudreau PN (2000) Two-component signal transduction. Annu Rev Biochem 69:183–215
Braun Y, Smirnova AV, Weingart H, Schenk A, Ullrich MS (2007) A temperature-sensing histidine kinase: function, genetics, and membrane topology. Methods Enzymol 423:222–249
Hunger K, Beckering CL, Marahiel MA (2004) Genetic evidence for the temperature-sensing ability of the membrane domain of the Bacillus subtilis histidine kinase DesK. FEMS Microbiol Lett 230:41–46
Suzuki I, Kanesaki Y, Mikami K, Kanehisa M, Murata N (2001) Cold-regulated genes under control of the cold sensor Hik33 in Synechocystis. Mol Microbiol 40:235–244
Mikami K, Kanesaki Y, Suzuki I, Murata N (2002) The histidine kinase Hik33 perceives osmotic stress and cold stress in Synechocystis sp PCC 6803. Mol Microbiol 46:905–915
Budde IP, Ullrich MS (2000) Interactions of Pseudomonas syringae pv. glycinea with host and nonhost plants in relation to temperature and phytotoxin synthesis. Mol Plant Microbe Interact 13:951–961
Mittal S, Davis KR (1995) Role of the phytotoxin coronatine in the infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Mol Plant Microbe Interact 8:165–171
Ullrich M, Penaloza-Vazquez A, Bailey AM, Bender CL (1995) A modified two-component regulatory system is involved in temperature-dependent biosynthesis of the Pseudomonas syringae phytotoxin coronatine. J Bacteriol 177:6160–6169
Smirnova AV, Braun Y, Ullrich MS (2008) Site-directed mutagenesis of the temperature-sensing histidine protein kinase CorS from Pseudomonas syringae. FEMS Microbiol Lett 283:231–238
Rangaswamy V, Bender CL (2000) Phosphorylation of CorS and CorR, regulatory proteins that modulate production of the phytotoxin coronatine in Pseudomonas syringae. FEMS Microbiol Lett 193:13–18
Guschina IA, Harwood JL (2006) Mechanisms of temperature adaptation in poikilotherms. FEBS Lett 580:5477–5483
Mansilla MC, Cybulski LE, Albanesi D, de Mendoza D (2004) Control of membrane lipid fluidity by molecular thermosensors. J Bacteriol 186:6681–6688
Aguilar PS, Hernandez-Arriaga AM, Cybulski LE, Erazo AC, de Mendoza D (2001) Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J 20:1681–1691
Albanesi D, Mansilla MC, de Mendoza D (2004) The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator. J Bacteriol 186:2655–2663
Cybulski LE, del Solar G, Craig PO, Espinosa M, de Mendoza D (2004) Bacillus subtilis DesR functions as a phosphorylation-activated switch to control membrane lipid fluidity. J Biol Chem 279:39340–39347
Mansilla MC, de Mendoza D (2005) The Bacillus subtilis desaturase: a model to understand phospholipid modification and temperature sensing. Arch Microbiol 183:229–235
Los DA, Murata N (1999) Responses to cold shock in cyanobacteria. J Mol Microbiol Biotechnol 1:221–230
Suzuki I, Los DA, Kanesaki Y, Mikami K, Murata N (2000) The pathway for perception and transduction of low-temperature signals in Synechocystis. EMBO J 19:1327–1334
Kanesaki Y, Yamamoto H, Paithoonrangsarid K, Shoumskaya M, Suzuki I, Hayashi H, Murata N (2007) Histidine kinases play important roles in the perception and signal transduction of hydrogen peroxide in the cyanobacterium, Synechocystis sp. PCC 6803. Plant J 49:313–324
Mikami K, Murata N (2003) Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog Lipid Res 42:527–543
Horvath I, Glatz A, Varvasovszki V, Torok Z, Pali T, Balogh G, Kovacs E, Nadasdi L, Benko S, Joo F, Vigh L (1998) Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proc Natl Acad Sci USA 95:3513–3518
Horvath I, Multhoff G, Sonnleitner A, Vigh L (2008) Membrane-associated stress proteins: more than simply chaperones. Biochim Biophys Acta 1778:1653–1664
Tuominen I, Pollari M, Tyystjarvi E, Tyystjarvi T (2006) The SigB sigma factor mediates high-temperature responses in the cyanobacterium Synechocystis sp. PCC6803. FEBS Lett 580:319–323
Nara T, Lee L, Imae Y (1991) Thermosensing ability of Trg and Tap chemoreceptors in Escherichia coli. J Bacteriol 173:1120–1124
Mizuno T, Imae Y (1984) Conditional inversion of the thermoresponse in Escherichia coli. J Bacteriol 159:360–367
Maeda K, Imae Y (1979) Thermosensory transduction in Escherichia coli: inhibition of the thermoresponse by L-serine. Proc Natl Acad Sci USA 76:91–95
Salman H, Libchaber A (2007) A concentration-dependent switch in the bacterial response to temperature. Nat Cell Biol 9:1098–1100
Nishiyama S, Maruyama IN, Homma M, Kawagishi I (1999) Inversion of thermosensing property of the bacterial receptor Tar by mutations in the second transmembrane region. J Mol Biol 286:1275–1284
Nishiyama SI, Umemura T, Nara T, Homma M, Kawagishi I (1999) Conversion of a bacterial warm sensor to a cold sensor by methylation of a single residue in the presence of an attractant. Mol Microbiol 32:357–365
Nishiyama S, Nara T, Homma M, Imae Y, Kawagishi I (1997) Thermosensing properties of mutant aspartate chemoreceptors with methyl-accepting sites replaced singly or multiply by alanine. J Bacteriol 179:6573–6580
Fischetti VA, Jones KF, Hollingshead SK, Scott JR (1988) Structure, function, and genetics of streptococcal M protein. Rev Infect Dis 10(Suppl 2):S356–S359
Cedervall T, Johansson MU, Akerstrom B (1997) Coiled-coil structure of group A streptococcal M proteins. Different temperature stability of class A and C proteins by hydrophobic–nonhydrophobic amino acid substitutions at heptad positions a and d. Biochemistry 36:4987–4994
Winter J, Jakob U (2004) Beyond transcription—new mechanisms for the regulation of molecular chaperones. Crit Rev Biochem Mol Biol 39:297–317
McCarty JS, Walker GC (1991) DnaK as a thermometer: threonine-199 is site of autophosphorylation and is critical for ATPase activity. Proc Natl Acad Sci USA 88:9513–9517
Grimshaw JP, Jelesarov I, Schönfeld HJ, Christen P (2001) Reversible thermal transition in GrpE, the nucleotide exchange factor of the DnaK heat-shock system. J Biol Chem 276:6098–6104
Harrison C (2003) GrpE, a nucleotide exchange factor for DnaK. Cell Stress Chaperones 8:218–224
Gelinas AD, Toth J, Bethoney KA, Langsetmo K, Stafford WF, Harrison CJ (2003) Thermodynamic linkage in the GrpE nucleotide exchange factor, a molecular thermosensor. Biochemistry 42:9050–9059
Siegenthaler RK, Christen P (2006) Tuning of DnaK chaperone action by nonnative protein sensor DnaJ and thermosensor GrpE. J Biol Chem 281:34448–34456
Groemping Y, Reinstein J (2001) Folding properties of the nucleotide exchange factor GrpE from Thermus thermophilus: GrpE is a thermosensor that mediates heat shock response. J Mol Biol 314:167–178
Groemping Y, Klostermeier D, Herrmann C, Veit T, Seidel R, Reinstein J (2001) Regulation of ATPase and chaperone cycle of DnaK from Thermus thermophilus by the nucleotide exchange factor GrpE. J Mol Biol 305:1173–1183
Schroda M, Vallon O, Whitelegge JP, Beck CF, Wollman FA (2001) The chloroplastic GrpE homolog of Chlamydomonas: two isoforms generated by differential splicing. Plant Cell 13:2823–2839
Willmund F, Muhlhaus T, Wojciechowska M, Schroda M (2007) The NH2-terminal domain of the chloroplast GrpE homolog CGE1 is required for dimerization and cochaperone function in vivo. J Biol Chem 282:11317–11328
Narberhaus F (2002) Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev 66:64–93
Haslbeck M, Franzmann T, Weinfurtner D, Buchner J (2005) Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol 12:842–846
Franzmann TM, Menhorn P, Walter S, Buchner J (2008) Activation of the chaperone Hsp26 is controlled by the rearrangement of its thermosensor domain. Mol Cell 29:207–216
Clausen T, Southan C, Ehrmann M (2002) The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell 10:443–455
Spiess C, Beil A, Ehrmann M (1999) A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339–347
Krojer T, Garrido-Franco M, Huber R, Ehrmann M, Clausen T (2002) Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature 416:455–459
Krojer T, Sawa J, Schafer E, Saibil HR, Ehrmann M, Clausen T (2008) Structural basis for the regulated protease and chaperone function of DegP. Nature 453:885–890
Kim DY, Kwon E, Shin YK, Kweon DH, Kim KK (2008) The mechanism of temperature-induced bacterial HtrA activation. J Mol Biol 377:410–420
Kamath-Loeb AS, Gross CA (1991) Translational regulation of σ32 synthesis: requirement for an internal control element. J Bacteriol 173:3904–3906
Nagai H, Yuzawa H, Yura T (1991) Interplay of two cis-acting mRNA regions in translational control of σ32 synthesis during the heat shock response of Escherichia coli. Proc Natl Acad Sci USA 88:10515–10519
Yuzawa H, Nagai H, Mori H, Yura T (1993) Heat induction of σ32 synthesis mediated by mRNA secondary structure: a primary step of the heat shock response in Escherichia coli. Nucleic Acids Res 21:5449–5455
Balsiger S, Ragaz C, Baron C, Narberhaus F (2004) Replicon-specific regulation of small heat shock genes in Agrobacterium tumefaciens. J Bacteriol 186:6824–6829
White-Ziegler CA, Um S, Perez NM, Berns AL, Malhowski AJ, Young S (2008) Low temperature (23°C) increases expression of biofilm-, cold-shock- and RpoS-dependent genes in Escherichia coli K-12. Microbiology 154:148–166
Moll I, Afonyushkin T, Vytvytska O, Kaberdin VR, Bläsi U (2003) Coincident Hfq binding and RNase E cleavage sites on mRNA and small regulatory RNAs. RNA 9:1308–1314
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klinkert, B., Narberhaus, F. Microbial thermosensors. Cell. Mol. Life Sci. 66, 2661–2676 (2009). https://doi.org/10.1007/s00018-009-0041-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-009-0041-3