Abstract
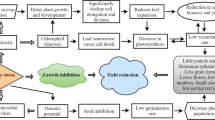
The identification of the sustainable approaches is required for the minimization of adverse impact of worldwide increasing soil salinity on plant growth, development, and productivity. This study investigated the protective role and major mechanism underlying salicylic acid (SA; 0.1, 0.5, or 1.0 mM)-induced glycine betaine (GB)-mediated tolerance to salinity (50 mM NaCl) in mungbean (Vigna radiata L. cultivar Punt Mung). The supply of 0.5 mM SA maximally increased the accumulation of GB (>40%) with respect to the control. This was further corroborated with the increase in water potential, antioxidant system (reduced glutathione (GSH), GSH/GSSG redox state, and glutathione reductase (GR) activity) and decreased Na+ and Cl− accumulation, Na+/K+ ratio, oxidative stress, and lipid peroxidation. This was also associated with the increased photosynthesis (14–18%) and growth (7–12%) parameters. Overall, SA-induced accumulation of GB protected photosynthesis and growth against 50 mM NaCl-accrued impacts in V. radiata through minimizing the accumulation of Na+ and Cl− ions, oxidative stress, and maintaining high GSH level that led to reduced cellular redox environment.







Similar content being viewed by others
References
Nazar, R., Iqbal, N., Masood, A., Syeed, S., & Khan, N. A. (2011). Understanding the significance of sulfur in improving salinity tolerance in plants. Environmental and Experimental Botany, 70(2–3), 80–87.
Fatma, M., Masood, A., Per, T. S., & Khan, N. A. (2016). Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Frontiers in Plant Science, 7, 52.
Ashraf, M., & Harris, P. J. C. (2004). Potential biochemical indicators of salinity tolerance in plants. Plant Science, 166(1), 3–16.
Wang, W. B., Kim, Y. H., Lee, H. S., Kim, K., & Kwask, S. S. (2009). Analysis of antioxidant enzymes activity during germination of alfalfa under salt and drought stresses. Plant Physiology and Biochemistry, 47(7), 570–577.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909–930.
Khan, N. A., Nazar, R., & Anjum, N. A. (2009). Growth, photosynthesis and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in ATP-sulfurylase activity under salinity stress. Scientia Horticulturae, 122(3), 455–460.
Fatma, M., & Khan, N. A. (2014). Nitric oxide protects photosynthetic capacity inhibition by salinity in Indian mustard. Journal of Functional and Environmental Botany, 4(2), 106–116.
Asgher, M., Per, T. S., Masood, A., Fatma, M., Freschi, L., Corpas, F. J., & Khan, N. A. (2017). Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environmental Science and Pollution Research, 24(3), 2273–2285.
Gunes, A., Alpaslan, A. I. M., Eraslan, G., Bagci, F. E., & Cicek, N. (2007). Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. Journal of Plant Physiology, 164(6), 728–736.
Nazar, R., Iqbal, N., Syeed, S., & Khan, N. A. (2011). Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. Journal of Plant Physiology, 168(8), 807–815.
Arfan, M., Athar, H. R., & Ashraf, M. (2007). Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress. Journal of Plant Physiology, 164(6), 685–694.
Syeed, S., Anjum, N. A., Nazar, R., Iqbal, N., Masood, A., & Khan, N. A. (2011). Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiologiae Plantarum, 33(3), 877–886.
Yang, W. J., Rich, P. J., Axtell, J. D., Wood, K. V., Bonham, C. C., Ejeta, G., & Rhodes, D. (2003). Genotypic variation for glycine betaine in sorghum. Crop Science, 43(1), 162–169.
Park, E. J., Jeknić, Z., Pino, M. T., Murata, N., & Chen, T. H. H. (2007). Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant, Cell & Environment, 30(8), 994–1005.
Chen, T. H., & Murata, N. (2008). Glycinebetaine: An effective protectant against abiotic stress in plants. Trends in Plant Science, 13(9), 499–505.
Ashraf, M., & Foolad, M. R. (2007). Roles of glycinebetaine and proline in improving plant abiotic stress tolerance. Environmental and Experimental Botany, 59(2), 206–216.
Hoque, M. A., Banu, M. N. A., Nakamura, Y., Shimoishi, Y., & Murata, Y. (2008). Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. Journal of Plant Physiology, 165(8), 813–824.
Islam, M. M., Hoque, M. A., Okuma, E., Banu, M. N. A., Shimoishi, Y., & Nakamura, Y. (2009). Exogenous proline and glycine betaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. Journal of Plant Physiology, 166(15), 1587–1597.
Hossain, M. A., & Fujita, M. (2010). Evidence for a role of exogenous glycine betaine and proline in antioxidant defense and methyl glyoxal detoxification systems in mung bean seedlings under salt stress. Physiology and Molecular Biology of Plants, 16(1), 19–29.
Murmu, K., Murmu, S., Kundu, C. K., & Bera, P. S. (2017). Exogenous proline and glycine betaine in plants under stress tolerance. International Journal of Current Microbiology and Applied Sciences, 6(9), 901–913.
Hussain, S. J., Masood, A., Anjum, N. A., & Khan, N. A. (2019). Sulfur-mediated control of salinity impact on photosynthesis and growth in mungbean cultivars screened for salt tolerance involves glutathione and proline metabolism, and glucose sensitivity. Acta Physiologiae Plantarum, 41, 129.
Williams, M., Senaratna, T., Dixon, K., & Sivasithamparam, K. (2003). Benzoic acid induces tolerance to biotic stress caused by Phytophthora cinnamomi in Banksai attenuate. Plant Growth Regulation, 41(1), 89–91.
Grieve, C. M., & Grattan, S. R. (1983). Rapid assay for determination of water soluble quaternary ammonium compounds. Plant and Soil, 70(2), 303–307.
Okuda, T., Masuda, Y., Yamanka, A., & Sagisaka, S. (1991). Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiology, 97(3), 265–1267.
Dhindsa, R. H., Plumb-Dhindsa, P., & Thorpe, T. A. (1981). Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. Journal of Experimental Botany, 32(1), 93–101.
Foyer, C. H., & Halliwell, B. (1976). The presence of glutathione and glutathione reductase in chloroplast: A proposed role in ascorbic acid metabolism. Planta, 133(1), 21–25.
Griffith, O. W. (1980). Determination of glutathione disulphide using glutathione reductase and 2-vinylpyridine. Analytical Biochemistry, 106(1), 207–212.
Khan, M. I. R., Iqbal, N., Masood, A., & Khan, N. A. (2012). Variation in salt tolerance of wheat cultivars: Role of glycinebetaine and ethylene. Pedosphere, 22(6), 746–754.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1), 1–15.
Hiscox, T. D. & and Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissues without maceration. Canadian Journal of Botany, 57, 1332-1334, 12.
Wutipraditkul, N., Wongwean, P., & Buaboocha, T. (2015). Alleviation of salt-induced oxidative stress in rice seedlings by proline and/or glycinebetaine. Biologia Plantarum, 599(3), 547–553.
Shabala, S., & Munns, R. (2017). Salinity stress: Physiological constraints and adaptive mechanisms. Plant stress physiology, 1(1), 24–63.
Iseki, K., Marubodee, R., Ehara, H., & Tomooka, N. (2017). A rapid quantification method for tissue Na+ and K+ concentrations in salt-tolerant and susceptible accessions in Vigna vexillata (L.). A. Rich. Plant Production Science, 20(1), 144–148.
Siefermann-Harms, D. (1987). The light-harvesting and protective functions of carotenoids in photosynthetic membranes. Physiologia Plantarum, 69(3), 561–568.
Li, T., Hu, Y., Du, X., Tang, H., Shen, C. & Wu, J. (2014). Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLOS One, 9(10), 109492.
Hussain, S. J., Khan, N. A., Anjum, N. A., Masood, A., & Khan, M. I. R. (2020). Mechanistic elucidation of salicylic acid and sulphur-induced defence systems, nitrogen metabolism, photosynthetic, and growth potential of mungbean (Vigna radiata) under salt stress. Journal of Plant Growth Regulation, 40, 1000–1016.
Khan, M. I. R., Asgher, M., & Khan, N. A. (2014). Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiology and Biochemistry, 80, 67–74.
Hamid, M., & Khalil-ur-Rehman, & Ashraf, M. Y. (2010). Salicylic acid–induced growth and biochemical changes in salt-stressed wheat. Communications in Soil Science and Plant Analysis, 41(4), 373–389.
Athar, H. U. R., Zafar, Z. U., & Ashraf, M. (2015). Glycinebetaine improved photosynthesis in canola under salt stress: Evaluation of chlorophyll fluorescence parameters as potential indicators. Journal of Agronomy and Crop Science, 201(6), 428–442.
Malekzadeh, P. (2015). Influence of exogenous application of glycinebetaine on antioxidative system and growth of salt-stressed soybean seedlings (Glycine max L.). Physiology and Molecular biology of Plants, 21(2), 225–232.
Rahman, M. S., Miyake, H., & Takeoka, Y. (2002). Effects of exogenous glycinebetaine on growth and ultrastructure of salt-stressed rice seedlings (Oryza sativa L.). Plant Production Science, 5(1), 33–44.
Jagendorf, A. T., & Takabe, T. (2001). Inducers of glycinebetaine synthesis in barley. Plant Physiology, 127(4), 1827–1835.
Lutts, S., Majerus, V., & Kinet, J. M. (1999). NaCl effects on proline metabolism in rice (Oryza sativa L.) seedlings. Physiologia Plantarum, 105(3), 450–458.
Shen, Y. G., Du, B. X., Zhang, W. K., Zhang, J. S., & Chen, S. Y. (2002). AhCMO, regulated by stresses in Atriplex hortensis, can improve drought tolerance in transgenic tobacco. Theoretical and Applied Genetics, 105(6-7), 815–821.
Misra, N., & Saxena, P. (2009). Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Science, 177(3), 181–189.
Abdelaal, K. A., Hafez, Y. M., El-Afry, M. M., Tantawy, D. S., & Alshaal, T. (2018). Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity, and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environmental Science and Pollution Research, 25(30), 30199–30211.
Anjum, N. A., Sofo, A., Scopa, A., Roychoudhury, A., Gill, S. S., Iqbal, M., Lukatkin, A. S., Pereira, E., Duarte, A. C., & Ahmad, I. (2015). Lipids and proteins - Major targets of oxidative modifications in abiotic stressed plants. Environmental Science and Pollution Research, 22(6), 4099–4121.
Munns, R., & Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59(1), 651–681.
Yang, Z., Yu, J., Merewitz, E., & Huang, B. (2012). Differential effects of abscisic acid and glycine betaine on physiological responses to drought and salinity stress for two perennial grass species. Journal of the American Society for Horticultural Science, 137(2), 96–106.
Rameeh, V., Gerami, M., Omran, V. G., & Ghavampour, S. (2017). Impact of glycine betaine on salinity tolerance of stevia (Stevia rebaudiana Bertoni) under in vitro condition. Cercetari Agronomice in Moldova, 50(3), 95–105.
Youssef, S. M., Elhady, S. A. A., Aref, R. M., & Riad, G. S. (2018). Salicylic acid attenuates the adverse effects of salinity on growth and yield and enhances peroxidase isozymes expression more competently than proline and glycine betaine in cucumber plants. Gesunde Pflanzen, 70(2), 75–90.
Lawson, T., & Vialet-Chabrand, S. (2019). Speedy stomata, photosynthesis and plant water use efficiency. New Phytologist, 221(1), 93–98.
Hamani, A. K. M., Li, S., Chen, J., Amin, A. S., Wang, G., Xiaojun, S., Zain, M., & Gao, Y. (2021). Linking exogenous foliar application of glycine betaine and stomatal characteristics with salinity stress tolerance in cotton (Gossypium hirsutum L.) seedlings. BMC Plant Biology 21, 146.
Chatrath, A., Mandal, P., & Anuradha, M. (2000). Effect of secondary salinization on photosynthesis in fodder oat (Avena sativa L.) genotypes. Journal of Agronomy and Crop Science, 184(1), 13–16.
Acknowledgements
Author (AM) acknowledges the Department of Science and Technology, Government of India, New Delhi for the grant SERB.LS-184/2014.
Author information
Authors and Affiliations
Contributions
Asim Masood: experimentation, methodology, writing original draft; Zebus Sehar: experimentation, data analyses; Naser Anjum: formal analysis, computational work; Shabina Syeed: data analyses, methodology; Nafees Khan: supervision, conceptualization.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Authors agreed to participate in this research.
Consent for Publication
All the authors have approved the last version of the manuscript for its submission/publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shabina Syeed, Zebus Sehar, and Asim Masood share first authorship.
Supplementary Information
ESM 1
(PDF 1081 kb)
Rights and permissions
About this article
Cite this article
Syeed, S., Sehar, Z., Masood, A. et al. Control of Elevated Ion Accumulation, Oxidative Stress, and Lipid Peroxidation with Salicylic Acid-Induced Accumulation of Glycine Betaine in Salinity-Exposed Vigna radiata L. Appl Biochem Biotechnol 193, 3301–3320 (2021). https://doi.org/10.1007/s12010-021-03595-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03595-9