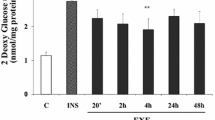
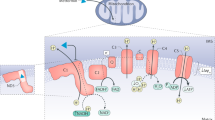
Abstract
The hypoglycemic sulfonylurea drugs cause reduction of blood glucose predominantly via stimulation of insulin release from pancreatic β cells. In addition, during long-term treatment, an insulin-independent blood glucose-decreasing mechanism is assumed to operate. This may include insulin-sensitizing and insulin-mimetic activity in muscle and adipose tissue. This review summarizes our current knowledge about the putative modes of action of the sulfonylurea compound, Amaryl, in pancreatic β cells and, in particular, peripheral target cells that form the molecular basis for its characteristic pharmacological and clinical profile. The analysis was performed in comparison with the conventional and the “golden standard” sulfonylurea, glibenclamide. I conclude: (I) The blood glucose decrease provoked by Amaryl can be explained by a combination of stimulation of insulin release from the pancreas and direct enhancement, as well as potentiation of the insulin response of glucose utilization in peripheral tissues only. (II) The underlying molecular mechanisms seemed to rely on β cells on a sulfonylurea receptor protein, SURX, associated with the ATP-sensitive potassium channel (KATP) and different from SUR1 for glibenclamide, and in muscle and adipose cells on: (a) the increased production of diacylglycerol and activation of protein kinase C; (b) the enhanced expression of glucose transporter isoforms; and (c) the insulin receptor-independent activation of the insulin receptor substrate/phosphatidylinositol-3-kinase pathway. (III) The latter mechanism involved a nonreceptor tyrosine kinase and a number of components, such as caveolin and glycosylphosphatidylinositol structures, which are assembled in caveolae/detergent-insoluble glycolipid-enriched rafts of the target cell plasma membrane. Since hyperinsulinism and permanent KATP closure are supposed to negatively affect the pathogenesis and therapy of non-insulin-dependent diabetes mellitus, the demonstrated higher insulin-independent blood glucose-lowering activity of Amaryl may be therapeutically relevant.












Similar content being viewed by others
References: (space limitation necessitated restriction of citations to reviews and studies directly related to Amaryl action which provide experimental details, more intense discussions of specific items and additional references)
Lebovitz HE. (1990) Oral hypoglycemic agents. In: Rifkin H, Porte D. (eds.) Ellenberg and Rifkin’s Diabetes mellitus. Theory and Practise. Elsevier, New York, pp. 554–574.
Ashcroft SJH, Ashcroft FM. (1992) The sulfonylurea receptor. Biochim. Biophys. Acta 1175: 45–59.
Philipson LH. (1995) ATP-sensitive K+ channels: paradigm lost, paradigm regained. Science 270: 1159.
Aguilar-Bryan L, Bryan J. (1999) Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 20: 101–135.
Tabuchi H, Yamamoto H, Matsumoto K, et al. (2000) Regulation of insulin secretion by overexpression of Ca2+/calmodulin-dependent protein kinase II in insulinoma MIN6 cells. Endocrinology 141: 2350–2360.
Easom RA. (1999) CaM kinase II: a protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes 48: 675–684.
Popoli M. (1993) Synaptotagmin is endogenously phosphorylated by Ca2+/calmodulin protein kinase II in synaptic vesicles. FEBS Lett. 317: 85–88.
Matsumoto K, Ebihara K, Yamamoto H, et al. (1999) Cloning from insulinoma cells of synapsin I associated with insulin secretory granules. J. Biol. Chem. 274: 2053–2059.
Mshlig M, Wolter S, Mayer P, et al. (1997) Insulinoma cells contain an isoform of Ca2+/calmodulin-dependent protein kinase IIδ associated with insulin secretion vesicles. Endocrinology 138: 2577–2584.
Skeer JM, Degano P, Coles B, Potier M, Ashcroft FM, Ashcroft SJH. (1994) Determination of the molecular mass of the native beta-cell sulfonylurea receptor. FEBS Lett. 338: 98–102.
Bryan J, Aguilar-Bryan L. (1999) Sulfonylurea receptors: ABC transporters that regulate ATP-sensitive K+ channels. Biochim. Biophys. Acta 1461: 285–303.
Clement IV JP, Kunjilwar K, Gonzalez G, et al. (1997) Association and stoichiometry of KATP channel subunits. Neuron 18: 827–838.
Bryan LA, Nichols CG, Wechsler SW, et al. (1995) Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268: 423–426.
Inagaki N, Gonoi T, Clement JP, et al. (1996) Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 270: 1167–1170.
Ueda K, Komine J, Matsuo M, Seino S, Amachi T. (1999) Cooperative binding of ATP and MgADP in the sulfonylurea receptor is modulated by glibenclamide. Proc. Natl. Acad. Sci. USA 96: 1268–1272.
Babenko AP, Aguilar-Bryan L, Bryan J. (1998) A view of SUR/KIR6.X, KATP channels. Annu. Rev. Physiol. 60: 667–687.
Ashfield R, Gribble FM, Ashcroft SJ, Ashcroft FM. (1999) Identification of the high-affinity tolbutamide site on the SUR1 subunit of the K(ATP) channel. Diabetes 48: 1341–1347.
Babenko AP, Gonzalez G, Bryan J. (1999) The tolbutamide site of SUR1 and a mechanism for its functional coupling to KATP channel closure. FEBS Lett. 459: 367–376.
Aguilar-Bryan L, Clement JP, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. (1998) Toward understanding the assembly and structure of KATP channels. Physiol. Rev. 78: 227–245.
Uhde I, Toman A, Gross I, Schwanstecher C, Schwanstecher M. (1999) Identification of the potassium channel opener site on sulfonylurea receptors. J. Biol. Chem. 274: 28079–28082.
Thomas PM, Cote GJ, Wohlik N, et al. (1995) Mutations in the sulfonylurea receptor gene in familial hyperinsulinemic hypoglycemia of infancy. Science 268: 426–429.
Thomas P, Ye Y, Lightner E. (1996) Mutations of the pancreatic islet inward rectifier also lead to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum. Mol. Gen. 5: 1809–1812.
Kane C, Shepherd RM, Squires PE, et al. (1996) Loss of functional KATP channels in pancreatic β cells causes persistent hyperinsulinemic hypoglycemia of infancy. Nature Med. 2: 1344–1347.
Gribble FM, Tucker SJ, Ashcroft FM. (1997) The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by MgADP and diazoxide. EMBO J. 16: 1145–1152.
Nichols CG, Shyng S-L, Nestorowicz A. (1996) Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272: 1785–1787.
Shyng S-L, Ferrigni T, Sheppard JB. (1998) Functional analysis of novel mutations in the sulfonylurea receptor 1 associated with persistent hyperinsulinemic hypoglycemia of infancy. Diabetes 47: 1145–1151.
Müller G, Hartz D, Pünter J, Ökonomopulos R, Kramer W. (1994) Differential interaction of glimepiride and glibenclamide with the β-cell sulfonylurea receptor: I. Binding characteristics. Biochim. Biophys. Acta 1191: 267–277.
Kramer W, Müller G, Girbig F, et al. (1995) The molecular interaction of sulfonylureas with β-cell ATP-sensitive K+-channels. Diabetes Res. Clin. Pract. 28(Suppl.): S67–S80.
Kramer W, Müller G, Girbig F, et al. (1994) Differential interaction of glimepiride and glibenclamide with the β-cell sulfonylurea receptor: II. Photoaffinity labeling of a 65-kDa protein with [3H]glimepiride. Biochim. Biophys. Acta 1191: 278–290.
Kramer W, Geisen K, Müller G. (1996) Characterization of the molecular mode of action of the sulfonylurea, glimepiride, at pancreatic β-cells. Horm. Metab. Res. 28: 464–468.
Kramer W, Ökonomopulos R, Pünter J, Summ H-D. (1988) Direct photoaffinity labeling of the putative sulfonylurea receptor in rat β-cell tumor membranes by [3H]glibenclamide FEBS Lett. 229: 355–359.
Ashcroft FM. (1996) Mechanisms of the glycaemic effects of sulfonylureas. Horm. Metab. Res. 28: 456–463.
Ashcroft FM, Gribble FM. (1999) ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia 42: 903–919.
Draeger E. (1995) Clinical profile of glimepiride. Diabetes Res. Clin. Pract. 28: S139–S146.
Langtry HD, Balfour JA. (1998) Glimepiride—a review of its pharmacological and clinical efficacy in the management of type 2 diabetes mellitus. Drugs 55: 563–584.
Tsumura K. (1995) Clinical evaluation of glimepiride (HOE490) in NIDDM, including a double blind comparative study versus gliclazide. Diabetes Res. Clin. Pract. 28: S147–S149.
Dills DG, Schneider J, Glimepiride/Glyburide Research Group. (1996) Clinical evaluation of glimepiride versus glyburide in NIDDM in a double-blind comparative study. Horm. Metab. Res. 28: 426–429.
Draeger KE, Wernicke-Panten K, Lomp HJ. (1996) Long-term treatment of type 2 diabetic patients with a new oral anti-diabetic agent glimepiride (Amaryl): a double-blind comparison with glibenclamide. Horm. Metab. Res. 28: 419–425.
Müller G, Satoh Y, Geisen K. (1995) Extrapancreatic effects of sulfonylureas — a comparison between glimepiride and conventional sulfonylureas. Diabetes Res. Clin. Pract. 28(Suppl.): S115–S137.
Müller G, Geisen K. (1996) Characterization of the molecular mode of action of the sulfonylurea, glimepiride, at adipocytes. Horm. Metab. Res. 28: 469–487.
Geisen K. (1988) Special pharmacology of the new sulfonylurea glimepiride. Drug. Res. 38: 1120–1130.
Müller G, Wied S, Wetekam E-M, Crecelius A, Unkelbach A, Pünter J. (1994) Stimulation of glucose utilization in 3T3 adipocytes and rat diaphragm in vitro by the sulfonylureas, glimepiride and glibenclamide, is correlated with modulations of the cAMP regulatory cascade. Biochem. Pharmacol. 48: 985–996.
Davidson MB, Molnar G, Furman A, Yamaguchi D. (1991) Glyburide-stimulated glucose transport in cultured muscle cells via protein kinase C-mediated pathway requiring new protein synthesis. Diabetes 40: 1531–1538.
Rogers BJ, Standaert ML, Pollet RJ. (1987) Direct effects of sulfonylurea agents on glucose transport BC3H-1 myocyte. Diabetes 36: 1292–1296.
Bak JF, Schmitz O, Sorensen NS, Pedersen O. (1989) Post-receptor effects of sulfonylurea on skeletal muscle glycogen synthase activity in type II diabetic patients. Diabetes 38: 1343–1350.
Jacobs DB, Jung CY. (1985) Sulfonylurea potentiates insulin-induced recruitment of glucose transport carrier in rat adipocytes. J. Biol. Chem. 260: 2593–2596.
Altan N, ALtan VM, Mikolay L, Larner J, Schwartz CFW. (1985) Insulin-like and insulin-enhancing effects of the sulfonylurea glyburide on rat adipose glycogen synthase. Diabetes 34: 281–286.
Jacobs DB, Jung CY. (1985) Sulfonylurea potentiates insulin-induced recruitment of glucose transport carrier in rat adipocytes. J. Biol. Chem. 260: 2593–2596.
Martz A, Jo I, Jung CY. (1988) Sulfonylurea binding to adipocyte membrane and potentiation of insulin stimulated hexose transport. J. Biol. Chem. 264: 13672–13678.
Maloff BL, Lockwood DH. (1981) In vitro effects of a sulfonylurea on insulin action in adipocytes. J. Clin. Invest. 68: 85–90.
Müller G, Wied S. (1993) The sulfonylurea drug, glimepiride, stimulates glucose transport, glucose transporter translocation, and dephosphorylation in insulin-resistant rat adipocytes in vitro. Diabetes 42: 1852–1867.
Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. (1999) Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. J. Biol. Chem. 274: 2593–2596.
Holman GD, Kasuga M. (1997) From receptor to transporter: insulin signalling to glucose transport. Diabetologia 40: 991–1003.
Czech MP, Corvera S. (1999) Signaling mechanisms that regulate glucose transport. J. Biol. Chem. 274: 1865–1868.
Bähr M, v. Holtey M, Müller G, Eckel J. (1995) Direct stimulation of myocardial glucose transport and glucose transporter (Glut1) and Glut4 protein expression by the sulfonylurea glimepiride. Endocrinology 136: 2547–2553.
Eckel J. (1996) Direct effects of glimepiride on protein expression of cardiac glucose transporters. Horm. Metab. Res. 28: 508–511
Gustafson TA, Moodie SA, Lavan BE. (1999) The insulin receptor and metabolic signaling. In: Blaustein, Greger, Grunicke, et al (eds.) Reviews in Physiology, Biochemistry and Pharmacology, vol. 137. Springer, Berlin, pp. 71–192.
White MF. (1997) The insulin signalling system and the IRS proteins. Diabetologia 40: S2–S17.
White MF. (1998) The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell. Biochem. 182: 3–11.
Coffer PJ, Jin J, Woodgett JR. (1998) Protein kinase B (c-Akt): a multifunctional mediator of phosphatidyl 3-kinase activation. Biochem. J. 335: 1–13.
Cohen P, Alessi DR, Cross DAE. (1997) PDK1, one of the missing links in insulin signal transduction? FEBS Lett. 410: 3–10.
White MF. (1998) The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell. Biochem. 182: 3–11.
Shepherd PR, Withers DJ, Siddle K. (1998) Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem. J. 333: 471–490.
Nystrom FH, Quon MJ. (1999) Insulin signalling: Metabolic pathways and mechanisms for specificity. Cell. Signal. 11: 563–574.
Araki E, Lipes MA, Patti ME, et al. (1994) Alternative pathway of insulin signaling in mice with targeted disruption of the IRS-1 gene. Nature 372: 186–190.
Tamemoto H, Kadowaki T, Tobe K, et al. (1994) Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372: 182–186.
Withers DJ, Gutierrez JS, Towery H, et al. (1998) Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391: 900–904.
Lavan BE, Fantin VR, Chang ET, Lane WS, Keller SR, Lienhard GE. (1997) A novel 160-kDa phosphotyrosine protein in insulin-treated embryonic kidney cells is a new member of the insulin receptor substrate family. J. Biol. Chem. 272: 21403–21407.
Liu SCH, Wang Q, Lienhard GE, Keller SR. (1999) Insulin receptor substrate 3 is not essential for growth or glucose homeostasis. J. Biol. Chem. 274: 18093–18099.
Takada Y, Takata Y, Iwanishi M, et al. (1996) Effect of glimepiride (HOE490) on insulin receptors of skeletal muscles from genetically diabetic KK-Ay mouse. Eur. J. Pharmacol. 308: 205–210.
Nosjean O, Briolay A, Roux B. (1997) Mammalian GPI proteins: sorting, membrane residence and functions. Biochim. Biophys. Acta 1331: 153–186.
Müller G, Wetekam E-A, Jung C, Bandlow W. (1994) Membrane association of lipoprotein lipase and a cAMP-binding ectoprotein in rat adipocytes. Biochemistry 33: 12149–12159.
Müller G, Dearey E-A, Pünter J. (1993) The sulfonylurea drug, glimepiride, stimulates release of glycosyl-phosphatidylinositolanchored plasma membrane proteins from 3T3 adipocytes. Biochem. J. 289: 509–521.
Saltiel AR, Fox JA, Sherline P, Cuatrecasas P. (1986) Insulin stimulates the generation from hepatic plasma membranes of modulators derived from an inositol glycolipid. Science 233: 967–972.
Romero GL, Luttrell L, Rogol A, Zeller K, Hewlett E, Larner J. (1988) Phosphatidylinositol-glycan anchors of membrane proteins: potential precursors of insulin mediators. Science 240: 509–511.
Müller G, Dearey E-A, Korndörfer A, Bandlow W. (1994) Stimulation of a glycosyl-phosphatidylinositol-specific phospholipase by insulin and the sulfonylurea, glimepiride, in rat adipocytes depends on increased glucose transport. J. Cell Biol. 126: 1267–1276.
Movahedi S, Hooper N. (1997) Insulin stimulates the release of the glycosyl phosphatidylinositol-anchored membrane dipeptidase from 3T3-L1 adipocytes through the action of a phospholipase C. Biochem. J. 326: 531–537.
Anderson RGW. (1998) The caveolae membrane system. Annu. Rev. Biochem. 67: 199–225.
Lisanti MP, Scherer PE, Tang ZL, Sargiacomo M. (1994) Caveolae, caveolin and caveolin-rich membrane domains: A signaling hypothesis. Trends Cell Biol. 4: 231–235.
Rothberg KG, Henser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RGW. (1992) Caveolin, a protein component of caveolae membrane coats. Cell 68: 673–682.
Parton RG. (1996) Caveolae and caveolins. Curr. Opin. Cell Biol. 8: 542–548.
Kurzchalia TV, Dupree P, Monier S. (1994) VIP-21 Caveolin, a protein of the trans-Golgi network and caveolae. FEBS Lett. 346: 88–91.
Das K, Lewis RY, Scherer PE, Lisanti MP. (1999) The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers. J. Biol. Chem. 274: 18721–18728.
Sargiacomo M, Scherer PE, Tang Z, Kübler E, Song KS, Sanders MC. (1995) Oligomeric structure of caveolin: Implications for caveolae membrane organizations. Proc. Natl. Acad. Sci. USA 92: 9407–9411.
Brown DA, London E. (1997) Breakthroughs and views. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 240: 1–7.
Okamoto T, Schlegel A, Scherer PE, Lisanti MP. (1998) Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 273: 5419–5422.
Schlegel A, Volonte D, Engelmann JA. (1999) Crowded little caves: structure and function of caveolae. Cell. Signal. 10: 457–463.
Müller G, Frick W. (1999) Signalling via caveolin: Involvement in the cross-talk between phosphoinositolglycans and insulin. Cell. Mol. Life Sci. 56: 945–970.
Li S, Couet J, Lisanti MP. (1996) Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J. Biol. Chem. 272: 29182–29190.
Couet J, Sargiacomo M, Lisanti MP. (1997) Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J. Biol. Chem. 272: 30429–30438.
Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. (1997) Identification of peptide and protein ligands for the caveolin-scaffolding domain. J. Biol. Chem. 272: 6525–6533.
Pulido N, Casla A, Suarez A, Casanova B, Arrieta FJ, Rovira A. (1996) Sulphonylurea stimulates glucose uptake in rats through an ATP-sensitive K+ channel dependent mechanism. Diabetologia 39: 22–27.
Shi H, Moustaid-Moussa N, Wilkison WO, Zemel MB. (1999) Role of the sulfonylurea receptor in regulating human adipocyte metabolism. FASEB J. 13: 1833–1838.
Rajan A, Luo Z-T, Kahn BB, Comstock JP, Cushman SW, Boyd III AE. (1994) Do adipocytes contain high affinity sulfonylurea receptors? Endocrinology 134: 1581–1588.
Draznin B, Sussman KE, Eckel RH, Kao M, Yost T, Sherman NA. (1988) Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperin-sulinemia. J. Clin. Invest. 82: 1848–1852.
Draznin B, Kao M, Sussman KE. (1987) Insulin and glyburide increase cytosolic free-Ca2+ concentration in isolated rat adipocytes. Diabetes 36: 174–177.
Kim JH, Kiefer LL, Woychik RP, et al. (1997) Agouti regulation of intracellular clacium. Role of melanocortin receptor. Am. J. Physiol. 272: E379–E384.
Zemel MB, Kim LL, Woychik RP, et al. (1995) Agouti regulation of intracellular calcium: role in the insulin resistance of viable yellow mice. Proc. Natl. Acad. Sci. U.S.A. 92: 4733–4737.
Gribble FM, Tucker SJ, Seino S, Ashcroft FM. (1998) Tissue specificity of sulphonylureas: studies on cloned cardiac and β-cell KATP channels. Diabetes 47: 1412–1418.
Smits P, Thien T. (1995) Cardiovascular effects of sulphonylurea derivatives. Implications for the treatment of NIDDM. Diabetologia 38: 116–122.
Leibowitz G, Cerasi E. (1996) Sulphonylurea treatment of NIDDM patients with cardiovascular disease: a mixed blessing? Diabetologia 39: 503–515.
UKPDS. (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with Type II diabetes (UKPDS 33). Lancet 352: 837–853.
Isomoto S, Kondo C, Yamada M. (1996) A novel sulphonylurea receptor forms with BIR (KIR6.2) a smooth muscle type of ATP-sensitive K+ channels. J. Biol. Chem. 271: 24321–24325.
Ämmälä C, Moorhouse A, Gribble FM. (1996) Promiscuous coupling between the sulphonylurea receptor and inwardly-rectifying potassium channels. Nature 379: 545–548.
Geisen K, Hitzel V, Ökonomopulos R, Pünter J, Weyer R, Summ H-D. (1985) Inhibition of [3H]glibenclamide binding to sulfonylurea receptors by oral antidiabetics. Drug Res. 35: 707–712.
Dunn-Meynell A, Rawson N, Levin B. (1998) Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res. 814: 41–54.
Spanswick D, Smith M, Groppi V, Logam S, Ashford ML. (1997) Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390: 521–525.
Harvey J, McKenna F, Herson PS, Spanswick D, Ashford ML. (1997) Leptin activates ATP-sensitive potassium channels in the rat insulin-secreting cell line, CRI-G1. J. Physiol. 504: 527–535.
Considine RV, Caro JF. (1997) Leptin and the regulation of body weight. Int. J. Biochem. Cell Biol. 29: 1255–1272.
Tartaglia LA. (1997) The leptin receptor. J. Biol. Chem. 272: 6093–6096.
Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. (2000) Central nervous system control of food intake. Nature 404: 661–671.
Harvey J, Ashford ML. (1998) Diazoxide- and leptin-activated K(ATP) currents exhibit differential sensitivity to englitazone and ciclazindol in the rat CRI-G1 insulin-secreting cell line. Brit. Pharmacol. 124: 1557–1565.
Harvey J, Ashford ML. (1998) Role of tyrosine phosphorylation in leptin activation of ATP-sensitive K+ channels in the rat insulinoma cell line CRI-G1. J. Physiol. 510: 47–61.
Harvey J, Ashford ML. (1998) Insulin occludes leptin activation of ATP-sensitive K+ channels in rat CRI-G1 insulin secreting cells. J. Physiol. 511: 695–706.
Mastick CC, Brady MJ, Saltiel AR. (1995) Insulin stimulates the tyrosine phosphorylation of caveolin. J. Cell Biol. 129: 1523–1531.
Saad MJA, Velloso LA, Carvalho CRO. (1995) Angiotensin II induces tyrosine phosphorylation of insulin receptor substrate 1 and its association with phosphatidylinositol 3-kinase in rat heart. Biochem. J. 310: 741–744.
Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. (1996) Gastrin stimulates tyrosine phosphorylation of insulin receptor substrate 1 and its association with Grb2 and the phosphatidylinositol 3-kinase. J. Biol. Chem. 271: 26356–26361.
Argetsinger LS, Hsu GW, Myers MG, Billestrup N, White MF, Carter-Su C. (1995) Growth hormone, interferon-γ, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J. Biol. Chem. 270: 14685–14692.
Verdier F, Chretien S, Billat C, Gisselbrecht S, Lacombe C, Mayeux P. (1997) Erythropoietin induces the tyrosine phosphorylation of insulin receptor substrate-2. J. Biol. Chem. 272: 26173–26178.
Lazar DF, Knez JJ, Medof ME, Cuatrecasas P, Saltiel AR. (1994) Stimulation of glycogen synthesis by insulin in human erythroleukemia cells requires the synthesis of glycosyl-phosphatidylinositol. Proc. Natl. Acad. Sci. USA 91: 9665–9669.
Jones DR, Varela-Nieto I. (1998) The role of glycosyl-phosphatidylinositol in signal transduction. Int. J. Biochem. Cell Biol. 30: 313–326.
Jones DR, Varela-Nieto I. (1999) Diabetes and the role of inositol-containing lipids in insulin signaling. Mol. Med. 5: 505–514.
Larner J, Huang LC. (1999) Identification of a novel inositol glycan signaling pathway with significant therapeutic relevance to insulin resistance: an insulin signaling model using both tyrosine kinase and G-proteins. Diabetes Rev. V7 N3: 217–231.
Frick W, Bauer A, Bauer J, Wied S, Müller G. (1998) Structure-activity relationship of synthetic phosphoinositolglycans mimicking metabolic insulin action. Biochemistry 38: 13421–13436.
Frick W, Bauer A, Bauer J, Wied S, Müller G. (1998) Insulin-mimetic signalling of synthetic phosphoinositolglycans in isolated rat adipocytes. Biochem. J. 336: 163–181.
Müller G, Wied S, Piossek C, Bauer A, Bauer J, Frick W. (1998) Convergence and divergence of the signaling pathways for insulin and phosphoinositolglycans. Mol. Med. 4: 299–323.
Müller G, Wied S, Frick W. (2000) Cross talk of pp125FAK and pp59Lyn non-receptor tyrosine kinases to insulin-mimetic signaling in adipocytes. Mol. Cell. Biol. 20: 4708–4723.
Badian M, Korn A, Lehr K-H. (1994) Absolute bioavailability of glimepiride (Amaryl registered) after oral administration. Drug Metab. Drug Interact. 11: 331–339.
Rosenkranz B, Profozic V, Metelko Z. (1996) Pharmacokinetics and safety of glimepiride at clinically effective doses in diabetic patients with renal impairment. Diabetologia 39: 1617–1624.
Lehr KH, Damm P. (1990) Simultaneous determination of the sulfonylurea glimepiride and its metabolites in human serum and urine by high-performance liquid chromatography after pre-column derivatization. J. Chromatogr. 526: 497–505.
Wernicke-Panten K, Haupt E, Pfeiffer C. (1994) Early onset of pharmacodynamic effects of glimepiride in type II diabetic patients [abstract]. Diabetologia 37(Suppl. 1): 163.
Rosenstock J, Samols E, Muchmore DB. (1996) Glimepiride, a new once-daily sulfonylurea: a double-blind placebo-controlled study of NIDDM patients. Diabetes Care 19: 1194–1199.
Sonnenberg GE, Garg DC, Weidler DJ. (1997) Short-term comparison of once-versus twice-daily administration of glimepiride in patients with non-insulin-dependent diabetes mellitus. Ann. Pharmacother. 31: 671–676.
Geisen K, Vegh A, Krause E. (1996) Cardiovascular effects of conventional sulfonylureas and glimepiride. Horm. Metab. Res. 28: 496–507.
Ballagi-Pordany G, Nemeth M, Aranyi Z. (1992) Effect of glimepiride on the electrical activity of isolated rabbit heart muscle. Drug Res. 42: 111–113.
Müller G, Ertl J, Gerl M, Preibisch G. (1997) Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J. Biol. Chem. 272: 10585–10593.
Müller G, Wied S, Crecelius A, Kessler A, Eckel J. (1997) Phosphoinositolglycan-peptides from yeast potently induce metabolic insulin actions in isolated rat adipocytes, cardiomyocytes, and diaphragms. Endocrinology 138: 3459–3475.
Grynkiewicz G, Pocnic M, Tsien RY. (1985) A new generation of Ca2+ indicators with greatly improved fluorescent properties. J. Biol. Chem. 260: 3440–3450.
Zerangue N, Schwappach B, Jan YN, Jan LY. (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22: 537–548.
Müller G, Wied S, Welte S. (2000) Involvement of caveolae in insulin-mimetic signaling by the sulfonylurea Amaryl. Chem. Phys. Lipids 7: 7–8 [abstract].
Acknowledgements
The following coworkers contributed to the biochemical and pharmacological studies with Amaryl during the past decade: A. Crecelius, E.-A. Dearey, K. Geisen, F. Girbig, U. Gutjahr, H. Gögelein, D. Hartz, C. Jung, S. Kowalewski, A. Korndörfer, W. Kramer, K.-H. Lehr, R. Okonomopulos, J. Pünter, A. Unkelbach, E.-M. Wetekam and S. Wied, (all Aventis Pharma Germany); M. Bähr, M. v. Holtey, and J. Eckel (all Diabetes Institute Düsseldorf Germany); M. Kobayashi (University of Tokayama, Japan); Y. Satoh and S. Shakuto (Aventis Pharma Ltd. Japan)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, G. The Molecular Mechanism of the Insulin-mimetic/sensitizing Activity of the Antidiabetic Sulfonylurea Drug Amaryl. Mol Med 6, 907–933 (2000). https://doi.org/10.1007/BF03401827
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401827