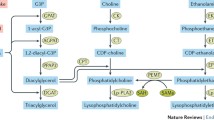
Abstract
Among metabolic diseases, diabetes is considered one of the most prevalent throughout the world. Currently, statistics show that over 10% of the world’s aged population (60 years and older) suffers from diabetes. As a consequence, it consumes a considerable proportion of world health expenditure. This review considers both past and current research into the molecular basis of insulin resistance found in type II diabetes and focuses on the role of inositol-containing phospholipid metabolism. It has been firmly established that the activation of phosphatidylinositol 3-kinase (PI3-K) is important for the propagation of the metabolic actions of insulin. In addition to the 3-phosphorylated phosphatidylinositols formed via the action of PI3-K, the glycosyl-phosphatidylinositol/inositol phosphoglycan (GPI/IPG) signaling component is also strongly implicated in mediating numerous metabolic actions of insulin. Although all the elements within the type II diabetes phenotype have not been fully defined, it has been proposed that defects in insulin transmembrane signaling through malfunction of inositol-containing phospholipid metabolism and absenteeism of the generation of phospholipid-derived second messengers may be associated with the appearance of the type II diabetic phenotype. Pharmaceutical approaches using synthetically produced IPG analogues, which themselves mimic insulin’s actions, alone or in combination with other drugs, may lead the way toward introducing alternative therapies for type II diabetes in the coming years.


Similar content being viewed by others
References
Jarret RJ, Keen H. (1976) Hyperglycemia and diabetes mellitus. Lancet 2: 1009–1012.
Bennet PH, Rushforth NB, Miller M, LeCompte PM. (1976) Epidemiological studies of diabetes in the Pima Indians. Recent Prog. Horm. Res. 32: 333–376.
National Diabetes Data Group. (1979) Classification and diagnosis of diabetes mellitus and other catagories of glucose intolerance. Diabetes 28: 1039–1057.
National Diabetes Advisory Board. (1984) Progress and promise in diabetes research. Department of Health and Human Services, Public Health Service, National Institutes of Health, Bethesda, MD.
Unger RH, Foster DW. (1985) Diabetes mellitus. In: Wilson JD, Foster DW (eds). Williams Textbook of Endocrinology. W.B. Saunders, Philadelphia, p. 1018–1080.
Eisenbarth GS, Connelly J, Soeldner JS. (1987) The “natural” history of type I diabetes. Diabetes Metab. Rev. 3: 873–891.
Lernmark A, Bärmeier H, Dube S, Hagopian W, Karlsen A, Wassmuth R. (1991) Autoimmunity of diabetes. Endocrinol. Metab. Clin. North Am. 20: 589–617.
Crisa L, Mordes JP, Rossini AA. (1992) Autoimmune diabetes mellitus in the BB rat. Diabetes Metab. Rev. 1: 4–37.
Bennett PH. (1983) Classification of diabetes. In: Ellenberg M, Rifkin H (eds). Diabetes Mellitus: Theory and Practice. Medical Examination Publishing, New Hyde Park, NY, p. 409–414.
Barnett AH, Eff C, Leslie RD, Pyke DA. (1981) Diabetes in identical twins. A study of 200 pairs. Diabetologia 20: 87–93.
Defronzo RA, Bonadonna RC, Ferrannini E. (1992) Pathogenesis of NIDDM: A balanced overview. Diabetes Care 15: 318–368.
Lavan BE, Lane WS, Lienhard GE. (1997) The 60-kDa phosphotyrosine protein in insulin-treated adipocytes is a new member of the insulin receptor substrate family. J. Biol. Chem. 272: 11439–11443.
Sciacchitano S, Taylor SI. (1997) Cloning, tissue expression and chromosomal localization of the mouse IRS-3 gene. Endocrinology 138: 4931–4940.
White MF, Yenush L. (1998) The IRS-signalling system: A network of docking proteins that mediate insulin and cytokine action. Curr. Top. Microbiol. Immunol. 228: 179–208.
Saltiel A, Cuatrecasas P. (1986) Insulin stimulates the generation from hepatic plasma membranes of modulators derived from an inositol glycolipid. Proc. Natl. Acad. Sci. U.S.A. 83: 5793–5797.
Stralfors P. (1997) Insulin second messengers. Bio Essays 19: 327–335.
Jones DR, Varela-Nieto I. (1998) The role of glycosyl-phosphatidylinositol in signal transduction. Int. J. Biochem. Cell. Biol 30: 313–326.
Larner J, Allan G, Kessler C, Reamer P, Gunn R, Huang LC. (1999) Phosphoinositol glycan-derived mediators and insulin resistance. Prospects for diagnosis and therapy. J. Basic Clin. Physiol. Pharmacol. 9: 127–137.
Macaulay SL, Larkins RG. (1990) Isolation of insulin-sensitive phosphatidylinositol-glycan from rat adipocytes. Biochem. J. 271: 427–435.
Sánchez-Arias JA, Sánchez-Gutierrez JC, Guadaño A, et al. (1992) Impairment of glycosyl-phosphatidylinositol-dependent insulin signaling system in isolated rat hepatocytes by streptzotocin-induced diabetes. Endocrinology 131: 1727–1733.
Farese RV, Standaert ML, Yamada K, et al. (1994) Insulin-induced activation of glycerol-3-phosphate acyltransferase by a chiro-inositol-containing insin mediator is defective in adipocytes of insulin-resistant, type II diabetic, Goto-Kakizaki rats. Proc. Natl. Acad. Sci. U.S.A. 91: 11040–11044.
Varela-Nieto I, Leon Y, Caro HN. (1996) Cell signalling by inositol phosphoglycan from different species. Comp. Biochem. Physiol. 115B: 223–241.
Fonteies MC, Huang LC, Larner J. (1996) Infusion of pH 2.0 d-chiro-inositol glycan putative mediator normalises plasma glucoase in streptozotocin diabetic rats at a doase equivalent to insulin without inducing hypoglycaemia. Diabetologia 39: 731–734.
Jones DR, Avila MA, Sanz C, Varela-Nieto I. (1997) Glycosyl-phosphatidylinositol-phospholipase type D: A possible candidate for the generation of second messengers. Biochem. Biophys. Res. Commun. 233: 432–437.
Villar AV, Goni FM, Alonso A, Jones DR, León Y, Varela-Nieto I. (1998) Phospholipase cleavage of glycosylphosphatidylinositol reconstituted in liposomal membranes. FEBS Lett. 432: 150–154.
Bruzik KS, Hakeem A, Tsai M-D. (1994) Are d-and l-chiro-phosphoinositides substrates of phosphatidylinositol-specific phospholipase C? Biochemistry 33: 8367–8374.
Larner J, Huang LC, Schwartz CFW, et al. (1988) Rat liver insulin mediator which stimulates pyruvate dehydrogenase phosphatase contains galactosamine and d-chiroinositol. Biochem. Biophys. Res. Commun. 151: 1416–1426.
Plourde R, d’Alarcao M, Saltiel AR. (1992) Synthesis and characterization of an insulin-mimetic phosphodisaccharide. J. Org. Chem. 57: 2606–2610.
Zapata A, Leon Y, Mato JM, Varela-Nieto I, Penades S, Martin-Lomas M. (1994) Synthesis and investigation of the possible insulin-like activity of 1-d-4-O- and l-d-6-O-(2-amino-2-deoxy-α-d-glucopyranosyl)-myo-inositol 1-phosphate and l-d-6-O-(2-amino-2-deoxy-α-d-glucopyranosyl)-myo-inositol 1, 2-(cyclic phosphate). Carbohydr. Res. 264: 21–31.
León Y, Sanz C, Giráldez F, Varela-Nieto I. (1998) Induction of cell growth by insulin and insulinlike growth factor-I is associated with jun expression in the otic vesicle. J. Comp. Neurol 398: 323–332.
Dietrich H, Espinosa JF, Chiara JL, et al. (1999) Glycosyl inositol derivatives related to inositol-phosphoglycan mediators: Synthesis, structure and biological activity. Chem. Eur. J. 5: 320–336.
Clémente R, Jones DR, Ochoa P, Romero G, Mato JM, Varela-Nieto I. (1995) Role of glycosyl-phosphatidylinositol hydrolysis as a mitogenic signal for epidermal growth factor. Cell Signal 7: 411–421.
Sekar MC. (1998) New developments in phosphoinositide signalling. Asian Pac. J. Pharmacol. 13: 51–63.
Reddy KK, Falck JR, Capdevila J. (1993) Insulin second messengers: Synthesis of 6-0-(2-amino-2-deoxy-α-d-glucopyranosyl)-d-chiro-inositol-1-phosphate. Tetrahedron Lett. 34: 7869–7872.
Leon Y, Sanz C, Frago LM, et al. (1999) Involvement of insulin-like growth factor-I in inner ear organogenesis and regeneration. Horm. Metab. Res. 31: 1–7.
Nestler JE, Jakuowicz DJ, Falcon de Vargas A, Brik C, Quintero N, Medina F. (1998) Insulin stimulates testosterone biosynthesis in human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J. Clin. Endocrinol. Metab. 83: 2001–2005.
Nestler JE. (1998) Inositolphosphoglycans (IPGs) as mediators of insulin’s steroidogenic actions. J. Basic Clin. Physiol. Pharmacol. 9: 197–204.
Frick W, Bauer A, Bauer J, Wied S, Müller G. (1998) Insulin-mimetic signalling of synthetic phosphinositolglycans in isolated rat adipocytes. Biochem. J. 336: 163–181.
Frick W, Bauer A, Bauer J, Wied S, Müller G. (1998) Structure-activity relationships of synthetic phosphoinositol glycans mimicking metabolic insulin action. Biochemistry 37: 13421–13436.
Kennington AS, Hill CR, Craig J, et al. (1990) Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N. Engl J. Med. 323: 373–378.
Asplin I, Galasko G, Larner J. (1993) Chiro-inositol deficiency and insulin resistance: A comparison of the chiro-inositol- and the myo-inositol-containing insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects. Proc. Natl. Acad. Sci. U.S.A. 90: 5924–5928.
Larner J, Craig JW. (1996) Urinary myo-inositol-to-chiro-inositol ratios and insulin resistance. Diabetes Care 19: 76–78.
Shashkin PN, Shaskina EF, Fernqvist-Forbes E, Zhou Y-P, Gull V, Katz A. (1997) Insulin mediators in man: Effects of glucose ingestion and insulin resistance. Diabetologia 40: 557–563.
Pak Y, Hong Y, Kim S, Piccariello T, Farese RV, Larner J. (1998) In vivo chiro-inositol metabolism in the rat: A defect in chiro-inositol synthesis from myo-inositol and an increased incorporation of chiro-[3H]inositol into phospholipid in the Goto-Kakizaki (G.K) rat. Mol. Cells 8: 301–309.
Varela-Nieto I, Alvarez L, Mato JM. (1993) Intracellular mediators of peptide hormone action: Glycosyl phosphatidylinositol/inositol phosphoglycan system. In: De Pablo F, Scanes CG (eds). Handbook of Endocrine Research Techniques. Academic Press, San Diego, p. 391.
Huang LC, Fonteies MC, Houston DB, Zhang C, Larner J. (1993) Chiroinositol deficiency and insulin resitance. III. Acute glycogenic and hypoglycemic effects of two inositol phosphoglycan insulin mediators in normal and strepozotocin-diabetic rats in vivo. Endocrinogy 132: 652–657.
Galasko GTF, Bao Y, Broomfield SJ, Hooper NM, Turner AJ, Larner J. (1995) Circulating factors and insulin resistance. I. A novel myoinositol 1,2-cyclic phosphate phosphoglycan insulin anatgonist from human plasma is elevated in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 80: 2419–2429.
Galasko GTF, Abe S, Lilley K, Zhang C, Larner J. (1996) Circulating factors and insulin resistance. H. The action of the novel myoinositol cyclic 1,2-inositol phosphate phosphoglycan insulin antagonist from human plasma in regulating pyruvate dehydrogenase phosphatase. J. Clin. Endocrinol. Metab. 81: 1051–1057.
Villalba M, Alvarez JF, Russell DS, Mato JM, Rosen OM. (1990) Hydrolysis of glycosyl-phosphatidylinositol in response to insulin is reduced in cells bearing kinase-deficient insulin receptors. Growth Factors 2: 91–97.
Suzuki S, Taneda Y, Hirai S, Yamamoto-Honda R, Toyota T. (1992) Mutated insulin receptor val 996 reduces insulin-dependent generation of inositol glycan and diacylglycerol. Diabetes 41: 1373–1379.
Kerouz NJ, Horsch D, Pons S, Kahn CR. (1997) Differential regulation of insulin receptor substrates-1 and −2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J. Clin. Invest. 100: 3164–3172.
Paz K, Hemi R, LeRoith D, et al. (1997) A molecular basis for insulin resistance. J. Biol. Chem. 272: 29911–29918.
Withers DJ, Ssnchez-Gutierrez J, Towery H, et al. (1998) Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391: 900–904.
Shepherd PR, Withers DJ, Siddle K. (1998) Phosphoinositide 3-kinase: The key switch in insulin signalling. Biochem. J. 333: 471–490.
Alessi DR, Downes CP. (1998) The role of PI 3-kinase in insulin action. Biochem. Biophys. Acta 1436: 151–164.
Anai M, Ono H, Funaki M, et al. (1998) Different subcellular distribution and regulation of expression of insulin receptor substrate (IRS)-3 from those of IRS-1 and IRS-2. J. Biol. Chem. 273: 29686–29692.
Kessler A, Müller G, Wied S, Crecelius A, Eckel J. (1998) Signalling pathways of an insulin-mimetic phoshoinositolglycan-peptide in muscle and adipose tissue. Biochem. J. 330: 277–286.
Müller G, Wied S, Crecelius A, Kessler A, Eckel J. (1997) Phosphoinositolglycan-peptides from yeast potently induce metabolic insulin actions in isolated rat adipocytes, cardiomyocytes and diaphragms. Endocrinology 138: 3459–3475.
Gustavsson J, Parpal S, Strålfors P. (1996) Insulin-stimulated glucose uptake involves the transition of glucose transporters to a caveloae-rich fraction within the plasma membrane: Implications for type II diabetes. Mol. Med. 2: 367–372.
Parpal S, Gustavsson J, Strålfors P. (1995) Isolation of phosphooligosaccharide/phosphoinositol glycan from caveolae and cytosol of insulin-stimulated cells. J. Cell. Biol. 131: 123–135.
Müller G, Wied S, Piossek C, Bauer A, Bauer J, Frick W. (1998) Convergence and divergence of the signalling pathways for insulin and phosphoi-nositolglycans. Mol. Med. 4: 299–323.
Misek DE, Saltiel AR. (1992) An inositol phosphate glycan derived from a Trypanosome brucei glycosyl-phosphatidylinositol mimics some of the metabolic actions of insulin. J. Biol. Chem. 267: 16266–16273.
Misek DE, Saltiel AR. (1994) An inositol phosphate glycan derived from a Trypanosome brucei glycosyl-phosphatidylinositol promotes protein dephosphorylation in rat epididymal adipocytes. Endocrinology 135: 1869–1876.
Abe S, Huang L, Larner J. (1996) Dephosphorylation of PDH by phosphoprotein phosphatases and its allosteric regulation by inositol glycans. Birkhäuser Verlag, Basel, p. 188.
Ortmeyer HK. (1998) Insulin increases liver protein phosphatase-1 and protein phosphatase-2C activities in lean, young adult rhesus monkeys. Horm. Metab. Res. 30: 705–710.
Huang LC, Heimark D, Linko J, Nolan R, Larner J. (1999) A model phosphatase 2C → phosphatase 1 activation cascade via dual control of inhibitor-1 (INH-1) and DARRP-32 dephosphorylation by two inositol glycan putative insulin mediators from beef liver. Biochem. Biophys. Res. Commun. 255: 150–156.
Proud CG, Denton RM. (1997) Molecular mechanisms for the control of translation by insulin. Biochem. J. 328: 329–341.
Lazar DF, Knez JJ, Medof ME, Cuatrecasas P, Saltiel AR. (1994) Stimulation of glycogen synthesis by insulin in human erythroleukemia cells requires the synthesis of glycosyl-phosphatidylinositol. Proc. Natl. Acad. Sci. U.S.A. 91: 9665–9669.
Kunjara K, Caro HN, McLean P, Rademacher TW. (1995) Tissue specific release of inositol phosphoglycans. In: Svati J (ed). Biopolymers and Bioproducts: Structure, Function and Applications. p. 301.
Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. (1995) Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J. Clin. Invest. 95: 2195–2204.
Bjornholm M, Kawano Y, Lehtihet M, Zierath JR. (1997) Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes 46: 524–527.
Rondinone CM, Wang LM, Lonnroth P, Wesslau C, Pierce JH, Smith U. (1997) Insulin receptor substrate (IRS) 1 is reduced and IRS-2 is the main docking protein for phosphatidylinositol 3-kinase in adipocytes from subjects with non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. U.S.A. 94: 4171–4175.
Acknowledgments
We thank Dr. J.E. Felm for critical reading of this manuscript and for helpful suggestions. D.R.J. holds a postdoctoral fellowship from the Association for International Cancer Research. The Department of Immunology and Oncology was founded and is supported by the C.S.I.C. and Pharmacia and Upjohn. This study was supported by grants from Dirección General de Investigation, Ciencia y Tecnología PM96-0075, and Europharma (Boehringer Ingelheim group) to I.V.-N.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, D.R., Varela-Nieto, I. Diabetes and the Role of Inositol-Containing Lipids in Insulin Signaling. Mol Med 5, 505–514 (1999). https://doi.org/10.1007/BF03401978
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401978