Abstract
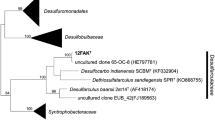
From granular sludge of an upflow anaerobic sludge bed (UASB) reactor treating paper-mill wastewater, a sulfate-reducing bacterium (strain ASRB1) was isolated with acetate as sole carbon and energy source. The bacterium was rod-shaped, (1.4–1.9×2.5–3.4 μm), nonmotile, and gram-negative. Optimum growth with acetate occurred around 37°C in freshwater medium (doubling time: 3.5–5.0 days). The bacterium grew on a range of organic acids, such as acetate, propionate, and butyrate, and on alcohols, and grew autotrophically with H2, CO2 and sulfate. Fastest growth occurred with formate, propionate, and ethanol (doubling time: approx. 1.5 days). Strain ASRB1 clusters with the delta subdivision of Proteobacteria and is closely related toSyntrophobacter wolinii a syntrophic propionate oxidizer. Strain ASRB1 was characterized as a new genus and species:Desulforhabdus amnigenus.
Similar content being viewed by others
References
Alphenaar PA, Visser A, Lettinga G (1993) The effect of liquid upward velocity and hydraulic retention time on granulation in UASB reactors treating wastewater with a high sulphate content. Bioresource Technol 43:249–258
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cashion P, Holder-Franklin MA, McCully J, Franklin M (1977) A rapid method for the base ratio determination of bacterial DNA. Anal Biochem 81:461–466
De Rijk P, Neefs JM, Van de Peer Y, De Wachter R (1992) Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res 20:2075–2089
Doetsch RN (1981) Determinative methods of light microscopy. In: Gerhardt P, Murray RGE, Costilow RN, Nester EW, Wood WA, Krieg NR, Phillips GB (eds) Manual of methods for general bacteriology. American Society for Microbiology, Washington DC, pp 21–33
Felsenstein J (1982) Numerical methods for inferring evolutionary trees. Q Rev Biol 57:379–404
Gujer W, Zehnder AJB (1983) Conversion processes in anaerobic digestion. Water Sci Technol 15:127–167
Harada H, Uemura S, Momonoi K (1994) Interaction between sulfate-reducing and methane-producing bacteria in UASB reactors fed with low strength wastes containing different levels of sulfate. Water Res 28:355–367
Harmsen HJM, Wullings B, Akkermans ADL, Ludwig W, Stams AJM (1993) Phylogenetic analysis ofSyntrophobacter wolinii reveals a relationship with sulfate-reducing bacteria. Arch Microbiol 160:238–240
Hungate RE (1969) A roll tube method for cultivation of strict anaerobes. In: Norris JR, Ribbons DW (eds) Methods in microbiology, vol 3b. Academic Press, New York London, pp 117–132
Isa Z, Grusenmeyer S, Verstraete W (1986) Sulfate reduction relative to methane production in high-rate anaerobic digestion: technical aspects. Appl Environ Microbiol 51:572–579
Jeris JS, McCarty PL (1965) The biochemistry of methane fermentation using C14 tracers. J Water Pollut Control Fed 37:178–192
Jetten MSM, Stams AJM, Zehnder AJB (1990) Acetate threshold values and acetate-activating enzymes in methanogenic bacteria. FEMS Microbiol Ecol 73:339–344
Jetten MSMS Stams AJM, Zehnder AJB (1992) Methanogenesis from acetate: a comparison of the acetate metabolism inMethanothrix soehngenii andMethanosarcina sp. FEMS Microbiol Rev 88:181–198
Kengen SWM, Stams AJM (1994) Formation ofl-alanine as a reduced end product in carbohydrate fermentation by the hyperthermophilic archeonPyrococcus furiosus. Arch Microbiol 161:168–175
Larsen N, Olsen GJ, Maidak BL, McCaughey MJ, Overbeek R, Macke TJ, Marsh TL, Woese CR (1993) The ribosomal database project. Nucleic Acids Res 21:3021–3023
Love CA, Patel BKC, Nichols PD, Stackebrandt E (1993)Desulfotomaculum australicum, sp. nov., a thermophilic sulfate-reducing bacterium isolated from the Great Artesian Basin of Australia. Syst Appl Microbiol 16:244–251
McCartney DM, Oleszkiewicz JA (1991) Sulfide inhibition of anaerobic degradation of lactate and acetate. Water Res 25:203–209
Mesbah M, Premachandran U, Whitman WB (1989) Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol 39: 159–167
Olsen GJ, Overbeek R, Larsen N, Marsh TL, McCaughey MJ, Maciukenas MA, Kuan WM, Macke TJ, Xing Y, Woese CR (1992) The ribosomal database project. Nucleic Acids Res 20: 2199–2200
Ostle AG, Holt JG (1982) Nile blue A as a fluorescent stain for poly-(-hydroxybutyrate. Appl Environ Microbiol 44:238–241
Oude Elferink SJWH, Visser A, Hulshoff Pol LW, Stams AJM, (1994) Sulfate reduction in methanogenic bioreactors. FEMS Microbiol Rev 15:119–136
Platen H, Temmes A, Schink B (1990) Anaerobic degradation of acetone byDesulfococcus biacutus sp. nov. Arch Microbiol 154:355–361
Postgate JR (1959) A diagnostic reaction ofDesulphovibrio desulphuricans. Nature 183:481–482
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schauder R, Eikmanns B, Thauer RK, Widdel F, Fuchs G (1986) Acetate oxidation to CO2 in anaerobic bacteria via a novel pathway not involving reactions of the citric acid cycle. Arch Microbiol 145:162–172
Smith PH, Mah RA (1966) Kinetics of acetate metabolism during sludge digestion. Appl Microbiol 14:368–371
Stams AJM, Van Dijk JB, Dijkema C, Plugge CM (1993) Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl Environ Microbiol 59:1114–1119
Szewzyk R, Pfennig N (1987) Complete oxidation of catechol by the strictly anaerobic sulfate-reducingDesulfobacterium catecholicum sp. nov. Arch Microbiol 147:162–168
Tamaoka J, Komagata K (1984) Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol Lett 25:125–128
Trüper HG, Schlegel HG (1964) Sulphur metabolism in Thiorhodaceae. 1. Quantitative measurements on growing cells ofChromatium okenii. Antonie Van Leeuwenhoek 30:225–238
Visser A, Beeksma I, Van der Zee F, Stams AJM, Lettinga G (1993a) Anaerobic degradation of volatile fatty acids at different sulphate concentrations. Appl Microbiol Biotechnol 40:549–556
Visser A, Alphenaar PA, Gao Y, Van Rossem G, Lettinga G (1993b) Granulation and immobilisation of methanogenic and sulfate-reducing bacteria in high-rate anaerobic reactors. Appl Microbiol Biotechnol 40:575–581
Wallrabenstein C, Hauschild E, Schink B (1994) Pure culture properties of ‘Syntrophobacter wolinii’. FEMS Microbiol Lett 123:249–254
Whitman WB, Bowen TL, Boone DR (1992) The methanogenic bacteria. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds) The prokaryotes, vol. 2. Springer, Berlin Heidelberg New York, pp 719–767
Widdel F (1980) Anaerober Abbau van Fettsäuren und Benzoesäure durch neue isolierte Arten Sulfat-reduzierender Bakterien. PhD thesis, University of Göttingen, Germany
Widdel F (1987) New types of acetate-oxidizing, sulfate-reducingDesulfobacter sp.,D. hydrogenophilus sp. nov.,D. latus sp. nov., andD. curvatus sp. nov. Arch Microbiol 148:286–291
Widdel F (1992) The genusDesulfotomaculum. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds) The prokaryotes, vol 2. Springer, Berlin Heidelberg New York, pp 1792–1799
Widdel F, Bak F (1992) Gram-negative mesophilic sulfate-reducting bacteria. In: Balows A, Trüper HG, Dworkin M., Harder W, Schleifer K-H (eds) The prokaryotes, vol 2. Springer, Berlin Heidelberg New York, pp 3352–3378
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oude Elferink, S.J.W.H., Maas, R.N., Harmsen, H.J.M. et al. Desulforhabdus amnigenus gen. nov. sp. nov., a sulfate reducer isolated from anaerobic granular sludge. Arch. Microbiol. 164, 119–124 (1995). https://doi.org/10.1007/BF02525317
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02525317