Abstract
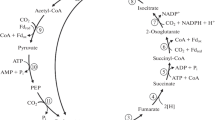
In several sulfate-reducing bacteria capable of complete oxidation of acetate (or acetyl CoA), the citric acid cycle is not operative. No 2-oxoglutarate dehydrogenase activity was found in these organisms, and the labelling pattern of oxaloacetate excludes its synthesis via 2-oxo-glutarate. These sulfate-reducers contained, however, high activities of the enzymes carbon monoxide dehydrogenase and formate dehydrogenase and catalyzed an isotope exchange between CO2 and the carboxyl group of acetate (or acetyl CoA), showing a direct C-C-cleavage of activated acetic acid. These findings suggest that in the investigated sulfate-reducers acetate is oxidized to CO2 via C1 intermediates. The proposed pathway provides a possible explanation for the reported different fluoroacetate sensitivity of acetate oxidation by anaerobic bacteria, for mini-methane formation, as well as for the postulated anaerobic methane oxidation by special sulfate-reducers.
Similar content being viewed by others
References
Alperin MJ, Reeburgh WS (1985) Inhibition experiments on anaerobic methane oxidation. Appl Environ Microbiol 50:940–945
Badziong W, Ditter B, Thauer RK (1979) Acetate and carbon dioxide assimilation by Desulfovibrio vulgaris (Marburg), growing on hydrogen and sulfate as sole energy sources. Arch Microbiol 123:301–305
Banat IM, Lindström EB, Nedwell DB, Balba MT (1981) Evidence for coexistence of two distinct functional groups of sulfate-reducing bacteria in salt marsh sediment. Appl Environ Microbiol 42:985–992
Bergmeyer HU (1974) Methoden der enzymatischen Analyse, 3rd ed. Verlag Chemie, Weinheim
Blakley RL (1969) The biochemistry of folic acid and related pteridines. North Holland Publishing Co, Amsterdam London
Brandis-Heep A, Gebhardt NA, Thauer RK, Widdel F, Pfennig N (1983) Anaerobic acetate oxidation to CO2 by Desulfobacter postgatei. 1. Demonstration of all enzymes required for the operation of the citric acid cycle. Arch Microbiol 136:222–229
Brysch K (1984) Kohlenstoffautotrophie bei sulfatreduzierenden Bakterien. Diplom thesis, Univ Konstanz
Cline JD (1969) Spectrophotometric determination of hydrogen-sulfide in natural waters. Limnol Oceanogr 14:454–458
Diekert G, Fuchs G, Thauer RK (1985) Properties and function of carbon monoxide dehydrogenase from anaerobic bacteria. In: Poole RK, Dow CS (eds) Microbial gas metabolism: Mechanistic, metabolic and biotechnological aspects. Academic Press, London, pp 115–130
Eikmanns B, Thauer RK (1984) Catalysis of an isotopic exchange between CO2 and the carboxyl group of acetate by Methanosarcina barkeri grown on acetate. Arch Microbiol 138:365–370
Eikmanns B, Fuchs G, Thauer RK (1985) Formation of carbon monoxide from CO2 and H2 in Methanobacterium thermoautotrophicum. Eur J Biochem 146:149–154
Evans MCW, Buchanan BB, Arnon DI (1966) A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci USA 55:928–934
Fuchs G, Stupperich E (1984) CO2 reduction to cell carbon in methanogens. In: Crawford RL, Hanson RS (eds) Microbial growth on C1-compounds. American Society for Microbiology, Washington D.C., pp 100–202
Fuchs G, Stupperich E (1986) Carbon assimilation pathways in Archaebacteria. J System Appl Microbiol (in press)
Fuchs G, Stupperich E, Jaenchen R (1980) Autotrophic CO2 fixation in Chlorobium limicola. Evidence against the operation of the Calvin cycle in growing cells. Arch Microbiol 128:56–63
Gebhardt NA, Linder D, Thauer RK (1983) Anaerobic acetate oxidation to CO2 by Desulfobacter postgatei. 2. Evidence from 14C-labelling studies for the operation of the citric acid cycle. Arch Microbiol 136:230–233
Gebhardt NA, Thauer RK, Linder D, Kaulfers PM, Pfennig N (1985) Mechanism of acetate oxidation to CO2 with elemental sulfur in Desulfuromonas acetoxidans. Arch Microbiol 141: 392–398
Gottschalk G (1968) The stereospecificity of the citrate synthase in sulfate-reducing and photosynthetic bacteria. Eur J Biochem 5:346–351
Hu SI, Drake HL, Wood HG (1982) Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and co-enzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol 149:440–448
Imhoff-Stuckle D, Pfennig N (1983) Isolation and characterization of a nicotinic acid-degrading sulfate-reducing bacterium, Desulfococcus niacini sp. nov. Arch Microbiol 136:194–198
Iversen N, Jørgensen BB (1985) Anaerobic methane oxidation rates at the sulfate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark). Limnol Oceanogr 30: 944–955
Jansen K, Thauer RK, Widdel F, Fuchs G (1984) Carbon assimilation pathways in sulfate reducing bacteria. Formate, carbon dioxide, carbon monoxide, and acetate by Desulfovibrio baarsii. Arch Microbiol 138:257–262
Krebs HA (1974) Cyclic processes in living matter. Enzymologia 12:88–100
Ljungdahl LG, Wood HG (1982) Acetate biosynthesis. In: Dolphin D (ed) B12, vol 2. Wiley, New York, pp 165–202
Lupton FS, Conrad R, Zeikus JG (1984) CO metabolism of Desulfovibrio vulgaris strain Madison: physiological function in the absence or presence of exogeneous subtrates. FEMS Microbiol Lett 23:263–268
Pfennig N, Widdel F, Trüper HG (1981) The dissimilatory sulfate-reducing bacteria. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes, vol 1. Springer, Berlin Heidelberg New York, pp 926–947
Phelps TJ, Conrad R, Zeikus JG (1985) Sulfate-dependent inter-species H2 transfer between Methanosarcina barkeri and Desulfovibrio vulgaris during coculture metabolism of acetate and methanol. Appl Environ Microbiol 50:589–594
Postgate JR (1969) Methane as a minor product of pyruvate metabolism by sulphate-reducing and other bacteria. J gen Microbiol 57:293–302
Postgate JR (1984) The sulphate-reducing bacteria. 2nd ed. Cambridge University Press
Ragsdale SW, Wood HG (1985) Acetate biosynthesis by acetogenic bacteria. Evidence that carbon monoxide dehydrogenase is the condensing enzyme that catalyzes the final steps of the synthesis. J Biol Chem 260:3970–3977
Scholtz R (1985) Untersuchungen zum Wachstum und zur CO2-Assimilation von neu isolierten autotrophen, sulfatreduzierenden Bakterien. Diplom thesis, Univ Ulm
Shiba H, Kawasumi T, Igarashi Y, Kodama T, Minoda Y (1985) The CO2 assimilation via the reductive tricarboxylic acid cycle in an obligately autotrophic, aerobic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus. Arch Microbiol 141:198–203
Simon H, Floss HG (1967) Anwendung von Isotopen in der organischen Chemie und Biochemie, vol 1. Springer, Berlin Heidelberg New York
Stupperich E, Fuchs G (1984) Autotrophic synthesis of activated acetic acid from two CO2 in Methanobacterium thermoautotrophicum. I. Properties of in vitro system. Arch Microbiol 139:8–13
Stupperich E, Hammel KE, Fuchs G, Thauer RK (1983) Carbon monoxide fixation into the carboxyl group of acetyl coenzyme A during autotrophic growth of Methanobacterium. FEBS Letters 152:21–23
Tabatabai MA (1974) Determination of sulfate in water sample. Sulphur Inst J 10:11–13
Thauer RK, Rupprecht E, Jungermann K (1970) Separation of 14C-formate from CO2 fixation metabolites by isionic exchange chromatography. Anal Biochem 38:461
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Widdel F (1986) Microbiology and ecology of sulfate- and sulfur-reducing bacteria. In: Zehnder AJB (ed) Environmental microbiology of anaerobes, chapt 10. John Wiley & Sons, New York London (in press)
Widdel F, Pfennig N (1977) A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol 112:119–122
Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov. sp. nov. Arch Microbiol 129:395–400
Widdel F, Pfennig N (1984) Dissimilatory sulfate- or sulfur-reducing bacteria. In: Krieg NR, Holt JG (eds) Bergey's manual of systematic bacteriology, IX ed, vol 1. William and Wilkins, Baltimore, pp 663–679
Widdel F, Kohring GW, Mayer F (1983) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limocola gen. nov. sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol 134:286–294
Yagi T (1959) Enzymic oxidation of carbon monoxide. II. J Biochem (Tokyo) 46:949–955
Zehnder AJB, Brock TD (1980) Anaerobic methane oxidation: Occurence and ecology. Appl Environ Microbiol 39:194–204
Zeikus JG, Fuchs G, Kenealy W, Thauer RK (1977) Oxido-reductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol 132:604–613
Zinder SH, Koch M (1984) Non-aceticlastic methanogenesis from acetate: acetate oxidation by a thermophilic synthrophic coculture. Arch Microbiol 138:263–272
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schauder, R., Eikmanns, B., Thauer, R.K. et al. Acetate oxidation to CO2 in anaerobic bacteria via a novel pathway not involving reactions of the citric acid cycle. Arch. Microbiol. 145, 162–172 (1986). https://doi.org/10.1007/BF00446775
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00446775