Abstract
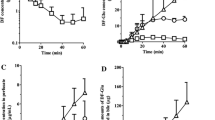
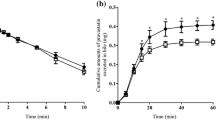
Single-pass and recirculating rat liver perfusion studies were conducted with [14C]enalapril and [3H] enalaprilat, a precursor-product pair, and the data were modeled according to a physiological model to compare the different biliary clearances for the solely formed metabolite, [14C]enalaprilat, with that of preformed [3H]enalaprilat. With single-pass perfusion, the apparent extraction ratio (or biliary clearance) of formed [14C]enalaprilat was 15-fold the extraction ratio of preformed [3H] enalaprilat, an observation attributed to the presence of a barrier for cellular entry of the metabolite. Upon recirculation of bolus doses of [14C]enalapril and [3H]enalaprilat, the biliary clearance, estimated conventionally as metabolite excretion rate/midtime metabolite concentration, for formed [14C]enalaprilat was again 10-to 15-fold higher than the biliary clearance for preformed [3H]enalaprilat, but this decayed with perfusion time and gradually approached values for preformed [3H]enalaprilat. The decreasing biliary clearance of formed enalaprilat with recirculation was explained by the dual contribution of the circulating and intrahepatic metabolite (formed from circulating drug) to excretion. Physiological modeling predicted (i) an influx barrier (from blood to cell) at the sinusoidal membrane as the rate-limiting process in the overall removal of enalaprilat, (ii) a 15-fold greater extraction ratio or biliary clearance for formed [14C]enalaprilat over [3H]enalaprilat during single-pass perfusion, and (iii) the time-dependent and declining behaviour of the biliary clearance for formed [14C]enalaprilat during recirculation of the medium. In the absence of a direct knowledge of eliminating organs in vivo, this variable pattern for excretory clearance of the formed metabolite within the organ is indicative of a metabolite formation organ.
Similar content being viewed by others
Abbreviations
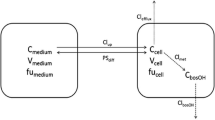
- C R :
-
denotes the reservoir concentration
- C In andC Out,L :
-
respectively, denote the input and output concentrations.
- Q L :
-
is the total hepatic plasma flow rate.
- Q Bile :
-
is the bile flow rate
- f p and fL :
-
denote the unbound fractions in plasma and liver tissue, respectively
- Cp :
-
is the concentration in renal plasma; CL is the concentration in liver;
- C Bile :
-
is the concentration in bile.
- v R,V p,V L, andV Bile :
-
denote the reservoir plasma, hepatic plasma, tissue, and bile volumes, respectively
- CL ind andCL efd :
-
denote the influx and efflux clearances, respectively
- CL mint,L ,L:
-
represents the hepatic metabolic intrinsic clearance of the drug
- CL bint,L L:
-
denotes the biliary intrinsic clearance
References
D. J. Tocco, F. A. De Luna, A. E. W. Duncan, T. C. Vassil, and E. H. Ulm. The physiological disposition and metabolism of enalapril maleate in laboratory animals.Drug Metab. Dispos. 10:15–19 (1982).
I. A. M. de Lannoy, R. Nespeca, and K. S. Pang. Renal handling of enalapril and its metabolite, enalaprilat, in the isolated red blood cell-perfused rat kidney.J. Pharmacol. Exp. Ther. 251:1211–1222 (1989).
I. A. M. de Lannoy, H. Hirayama, and K. S. Pang. A physiological model for renal drug metabolism: Enalapril esterolysis to enalaprilat in the isolated perfused rat kidney.J. Pharmacokin. Biopharm. 18:561–588 (1990).
K. S. Pang, W. F. Cherry, and E. H. Ulm. Disposition of enalapril in the intestine-liver preparation: Absorption, metabolism, and first-pass effects.J. Pharmacol. Exp. Ther. 233:788–795 (1985).
K. S. Pang, W. F. Cherry, J. A. Terrell, and E. H. Ulm. Disposition of enalapril and its diacid metabolite, enalaprilat, in a perfused rat liver preparation. Presence of a diffusional barrier into hepatocytes.Drug Metab. Dispos. 12:309–313 (1984).
K. S. Pang, W. F. Cherry, F. Barker, III, and C. A. Goresky. Esterases for enalapril hydrolysis are concentrated in the perihepatic venous region of the rat liver.J. Pharmacol. Exp. Ther. 257:294–301 (1991).
A. J. Schwab, F. Barker, III, C. A. Goresky, and K. S. Pang. Transfer of enalaprilat across rat liver cell membranes is barrier-limited.Am. J. Physiol. 258:G461-G475 (1990).
A. J. Schwab, I. A. M. de Lannoy, K. Poon, C. A. Goresky, and K. S. Pang. Enalaprilat handling by the rat kidney: barrier-limited cell entry.Am. J. Physiol. 263(Renal Fluid Electrolyte Physiol.32):F858-F869 (1992).
I. A. M. de Lannoy and K. S. Pang. Commentary: presence of a diffusional barrier on metabolite kinetics: Enalaprilat as a generatedversus preformed metabolite.Drug Metab. Dispos. 14:513–520 (1986).
I. A. M. de Lannoy and K. S. Pang. Effect of diffusional barriers on drug and metabolite kinetics.Drug Metab. Dispos. 15:51–58 (1987).
S. Miyauchi, Y. Sugiyama, T. Iga, and M. Hanano. Membrane-limited transport of the conjugative metabolites of 4-methylumbelliferone in rats.J. Pharm. Sci. 77:688–692 (1988).
J. W. Steele, B. Yagen, O. Hernandez, R. H. Cox, B. R. Smith, and J. R. Bend. The metabolism and excretion of styrene oxideglutathione conjugates in the rat and by isolated perfused liver, lung and kidney preparations.J. Pharmacol. Exp. Ther. 219:35–41 (1981).
L. A. Spry, T. V. Zenser, S. M. Cohen, and B. B. Davis. Role of renal metabolism and excretion in 5-nitrofuran-induced uroepithelial cancer in the rat.J. Clin. Invest. 76:1025–1031 (1985).
C. A. Goresky, K. S. Pang, A. J. Schwab, F. Barker, III, W. F. Cherry, and G. G. Bach. Uptake of a protein-bound, polar compound, acetaminophen sulfate, by perfused rat liver.Hepatology 15:173–190 (1992).
R. J. Henry, N. Chiamori, O. J. Golub, and S. Berkman. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic trans-aminase and lactic acid dehydrogenase.Am. J. Clin. Pathol. 34:381–398 (1960).
J. W. Severinghaus. Blood gas calculator.J. Appl. Physiol. 21:1108–1116 (1966).
R. M. Winslow, M.-L. Swenberg, R. L. Berger, R. I. Shrager, M. Luzzana, M. Samaja, and L. Rossi-Bernardi. Oxygen equilibrium curve of normal human blood and its evaluation by Adair's equation.J. Biol. Chem. 252:2331–2337 (1977).
K. S. Pang, W. F. Lee, W. F. Cherry, V. Yuen, J. Accaputo, S. Fayz, A. J. Schwab, and C. A. Goresky. Effects of perfusate flow rate on measured blood volume, Disse space, intracellular water spaces, and drug extraction in the perfused rat liver preparation: charac-terization by the technique of multiple indicator dilution technique.J. Pharmacokin. Biopharm. 16:595–632 (1988).
M. V. St-Pierre, W.-F. Lee, C. A. Goresky, and K. S. Pang. The multiple indicator dilution technique for characterization of normal and retrograde flow in once-through rat liver perfusions.Hepatology 9:285–296 (1989).
J. Reichen and G. Paumgartner. Excretory function of the liver. In N. B. Javitt (ed.),Liver and Biliary Tract Physiology I. International Review of Physiology, Vol. 21, University Park Press, Baltimore, 1980, pp. 103–150.
M. Rowland, L. Z. Benet, and G. G. Graham. Clearance concepts in pharmacokinetics.J. Pharmacokin. Biopharm. 1:123–136 (1977).
I. Bartosek, A. Guaitani, and S. Garattini. Long-term perfusion of isolated rat liver.Pharm-acology 8:244–258 (1972).
I. Bartosek, A. Guaitani, and L. L. Miller.Isolated Liver and Its Applications, Raven, New York, 1973.
B. D. Ross.Perfusion Techniques in Biochemistry. A Laboratory Manual, Clarendon, Oxford, 1972, pp. 135–257.
H. Schimassek. Perfusion of isolated rat liver with a semisynthetic medium and control of liver function.Life Sci. 11:629–634 (1962).
R. Hems, B. D. Ross, M. N. Berry, and H. A. Krebs. Gluconeogenesis in the perfused rat liver.Biochem. J. 101:284–292 (1966).
S. Keiding, H. Vilstrup, and L. Hansen. Importance of flow and haematocrit for metabolic function of perfused rat liver.Scand. J. Clin. Lab. Invest. 40:355–359 (1980).
K. S. Pang and J. R. Gillette. Complications in the estimation of hepatic blood flowin vivo by pharmacokinetic parameters: The area under the curve after the concomitant intravenous and intraperitoneal (or intraportal) dose of acetaminophen in rat.Drug Metab. Dispos. 6:567–576 (1978).
R. W. Brauer. Liver circulation and function.Physiol. Rev. 43:115–213 (1963).
G. R. Wilkinson and D. G. Shand. A physiological approach to hepatic drug clearance.Clin. Pharmacol. Ther. 18:377–390 (1975).
K. S. Pang and M. Rowland. Hepatic clearance of drugs. I. Theoretical consideration of a “well-stirred” model and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular activity on hepatic drug clearance.J. Pharma-cokin. Biopharm. 5:625–653 (1977).
I. A. M. de Lannoy and K. S. Pang. Combined recirculation of the rat liver and kidney: Studies with enalapril and enalaprilat.J. Pharmacokin. Biopharm. 21:423–456 (1993).
S. Miyauchi, Y. Sugiyama, H. Sato, Y. Sawada, I. Iga, and M. Hanano. Effect of a diffusional barrier to a metabolite across hepatocytes on its kinetics in “enzyme-distributed” models: A computer-aided simulation study.J. Pharmacokin. Biopharm. 15:4:399–421 (1987).
Author information
Authors and Affiliations
Additional information
This work was supported by the Medical Research Council of Canada. I. A. M. de Lannoy was a recipient of the Ontario Graduate Fellowship from the Ontario Ministry of Health; K. S. Pang was a recipient of the Faculty Development Award, Medical Research Council, Canada.
Rights and permissions
About this article
Cite this article
de Lannoy, I.A.M., Barker, F. & Pang, K.S. Formed and preformed metabolite excretion clearances in liver, a metabolite formation organ: Studies on enalapril and enalaprilat in the single-pass and recirculating perfused rat liver. Journal of Pharmacokinetics and Biopharmaceutics 21, 395–422 (1993). https://doi.org/10.1007/BF01061689
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01061689