Abstract
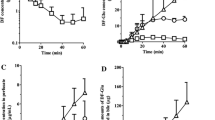
Combined recirculation of the rat liver (L) and kidney (IPK) at 10 ml min−1 per organ (LK) was developed to examine the hepatorenal handling of the precursor-metabolite pair: [14C]-enalapril and [3H]enalaprilat. Loading doses followed by constant infusion of [14C]enalapril and preformed [3H]enalaprilat to the reservoirs of the IPK or the LK preparation was used to achieve steady stale conditions. In both organs, enalapril was mostly metabolized to its dicarboxylic acid metabolite, enalaprilat, which was excreted unchanged. At steady state, the fractional excretion for [14C]enalapril (FE=0.45 to 0.48) and preformed [3H]enalaprilat (FE{pmi}=1.1) were constant and similar for both the IPK and LK. The additivity of clearance was demonstrated in the LK preparation, namely, the total clearance of enalapril was the sum of its hepatic and renal clearances. However, the apparent fractional excretion for fanned [14C]enalaprilat, FE{mi} and the apparent urinary clearance were time-dependent and higher than the corresponding values for preformed [3H]enalaprilat in both the IPK and LK. The FE{mi} and urinary clearance values further differed between the IPK and LK. Biliary clearance of formed vs. preformed enalaprilat displayed the same discrepant trends as observed for FE{mi} vs. FE{pmi} for the LK. These observations on the time-dependent and variable excretory clearance (urinary or biliary) of the formed metabolite vs. the constant, and much reduced, excretory clearance of the preformed metabolite are due to dual contributions to formed metabolite excretion: the nascently formed, intracellular metabolite which immediately underwent excretion and the formed metabolite which reentered the circulation, behaved as a preformed species. When data for the IPK and LK preparations were modeled with a physiological model with parameters previously reported for the L and IPK, all data, including metabolite excretory clearances, were well predicted. Model simulations revealed that the apparent FE{mi} differed between the LK and IPK preparations when the liver was present as an additional metabolite formation organ; the apparent excretory (urinary or
Similar content being viewed by others
Abbreviations
- k0 :
-
infusion rate into the reservoir
- CR :
-
reservoir concentration
- COut,k and COut,L :
-
venous concentrations for the kidney and liver
- Cp,k and cP,L :
-
concentrations in renal and hepatic plasma, respectively
- Ck and CL :
-
concentrations in kidney and liver tissue, respectively
- CU and CBile :
-
concentrations in urine and bile, respectively
- CL inb andCL ef b :
-
influx and efflux clearances, respectively, at the basolateral membrane of the renal tubular cell
- C inl and CL efl :
-
influx and efflux clearances, respectively, at the luminal membrane of the renal tubular cell
- CL mint,K :
-
renal metabolic intrinsic clearance of the drug
- CL ind and CL efd :
-
influx and efflux clearances, respectively, at the sinusoidal membrane
- CL m,Lint :
-
hepatic metabolic intrinsic clearance of the drug
- CL bint,L :
-
biliary intrinsic clearance
- VR :
-
plasma reservoir volume
- VP,K and VP,L :
-
plasma volumes of the kidney and liver, respectively
- VK and VL :
-
tissue volumes of the kidney and liver, respectively
- VU and VBile :
-
volumes of urine and bile, respectively
- QK and QL :
-
total renal and hepatic plasma flow rates, respectively
- GFR :
-
glomerular filtration rate
- QU and QBile :
-
urine and bile flow rates, respectively
- fP, fK, and fL :
-
unbound fractions in plasma and kidney and liver tissue, respectively
References
H. Daugaard, M. Egfjord, and K. Olgaard. Isolated perfused rat kidney and liver combined.Pflügers Arch. 409:220–222 (1987).
M. Yasuhara, H. Kataymaa, J. Fujiwara, K. Okumura, and R. Hori. Influence of acute renal failure on pharmacokinetics of phenosulfophthalein in rats: A comparative studyin vivo and in the simultaneous perfusion system of liver and kidney.J. Pharmacobiodyn. 8:377–384 (1985).
D. Alcorn, K. R. Emslie, B. D. Ross, G. B. Ryan, and J. D. Tange. Selective distal nephron damage during isolated kidney perfusion.Kidney Int. 19:638–647 (1981).
M. Brezis, S. Rosen, P. Silva, and F. H. Epstein. Selective vulnerability of the medullary thick ascending limb to anoxia in the isolated perfused rat kidney.J. Clin. Invest. 73:182–190 (1984).
K. S. Pang, W. F. Cherry, J. A. Terrell, and E. H. Ulm. Disposition of enalapril and its diacid metabolite, enalaprilat, in a perfused rat liver preparation. Presence of a diffusional barrier into hepatocytes.Drug Metab. Dispos. 12:309–313 (1984).
I. A. M. de Lannoy, R. Nespeca, and K. S. Pang. Renal handling of enalapril and enalaprilat: studies in the isolated red blood cell-perfused rat kidney.J. Pharmacol. Exp. Ther. 251:1211–1222 (1989).
I. A. M. de Lannoy, F. Barker, III, and K. S. Pang. Formed and preformed metabolite excretion clearances in liver, a metabolite formation organ. Studies with enalapril and enalaprilat in the single pass and recirculating perfused rat liver.J. Pharmacokin. Biopharm,21:395–422 (1993).
I. A. M. de Lannoy and K. S. Pang. Commentary: Presence of a diffusional barrier on metabolite kinetics: enalaprilat as a generatedversus preformed metabolite.Drug Metab. Dispos. 14:513–520 (1986).
A. J. Schwab, F. Barker, III, C. A. Goresky, and K. S. Pang. Transfer of enalaprilat across rat liver cell membranes is barrier-limited.Am. J. Physiol. 258:G461-G475 (1990).
A. J. Schwab, I. A. M. de Lannoy, K. Poon, C. A. Goresky, and K. S. Pang. Enalaprilat handling by the rat kidney: barrier-limited cell entry.Am. J. Physiol. 263:F858-F869 (1992).
B. D. Ross.Perfusion Techniques in Biochemistry. A Laboratory Manual, Clarendon, Oxford, 1972, pp. 135–257.
F. H. Epstein, J. T. Brosnan, J. D. Tange, and B. D. Ross. Improved function with amino acids in the isolated perfused kidney.Am. J. Physiol. 243:F284-F292 (1982).
R. J. Henry, N. Chiamori, O. J. Golub, and S. Berkman. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase and lactic acid dehydrogenase.Am. J. clin. Pathol. 34:381–398 (1960).
A. Heyrovsky. A new method for the determination of insulin in plasma and urine.Clin. Chim. Acta 1:470–474 (1956).
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall. Protein measurement with the folin phenol reagent.J. Biol. Chem. 193:265–275 (1951).
D. J. Tocco, F. A. de Luna, A. E. W. Duncan, T. C. Vassil, and E. H. Ulm. The physiological disposition and metabolism of enalapril maleate in laboratory animals.Drug Metab. Dispos. 10:15–19 (1982).
J. W. Severinghaus. Blood gas calculator.J. Appl. Physiol. 21:1108–1116 (1966).
R. M. Winslow, M.-L. Swenberg, R. L. Berger, R. I. Shrager, M. Luzzana, M. Samaja, and L. Rossi-Bernardi. Oxygen equilibrium curve of normal blood and its evaluation by Adair's equation.J. Biol. Chem. 225:2331–2337 (1977).
I. A. M. de Lannoy, H. Hirayama, and K. S. Pang. A physiological model for renal drug metabolism: Enalapril esterolysis to enalaprilat in the isolated perfused rat kidney.J. Pharmacokin. Biopharm. 18:561–588 (1990).
K. S. Pang, W. F. Cherry, and E. H. Ulm. Disposition of enalapril in the intestine-liver preparation: Absorption, metabolism, and first-pass effects.J. Pharmacol. Exp. Ther. 233:788–795 (1985).
E. Bojesen. The function of the urinary tract as a “dead space” in renal clearance experiments. Scand.J. Lab. Invest. 1:290–294 (1949).
P. Hekman and C. A. M. van Ginneken. Kinetic modeling of the renal excretion of iodopyracet in the dog.J. Pharmacokin. Biopharm. 10:77–92 (1982).
K. S. Pang, W. F. Cherry, V. Yuen, J. Accaputo, S. Fayz, W.-F. Lee, A. J. Schwab, and C. A. Goresky. Effects of perfusate flow rate on measured blood volume, Disse space, intracellular water spaces, and drug extraction in the perfused rat liver preparation: Characterization by the technique of multiple indicator dilution technique.J. Pharmacokin. Biopharm. 16:595–632 (1988).
M. V. St-Pierre, W.-F. Lee, A. J. Schwab, C. A. Goresky, and K. S. Pang. The multiple indicator dilution technique for characterization of normal and retrograde flow in oncethrough rat liver perfusions.Hepatology 9:285–296 (1989).
W. S. Spector.Handbook of Biological Data, W. B. Saunders, Philadelphia, PA, 1956, pp. 75; 341.
J. Reichen and G. Paumgartner. Excretory function of the liver. In N. B. Javitt (ed.),Liver and Biliary Tract Physiology I. International Review of Physiology, Vol. 21, University Park Press, MD, 1980, pp. 103–150.
E. D. Frohlich. Vascular effects of the Krebs intermediate metabolites.Am. J. Physiol. 208:149–153 (1965).
B. M. Brenner, T. W. Meyer, and T. H. Hostetter. Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease.New Engl. J. Med. 307:652–659 (1982).
M. Brezis, S. Rosen, J. S. Stoff, K. Spokes, P. Silva, P. Epstein. and F. H. Epstein. Inhibition of prostaglandin synthesis in rat kidney perfused with and without erythrocytes: Implication for analgesic nephropathy.Min. Electro. Metab. 12:326–332 (1986).
Author information
Authors and Affiliations
Additional information
This work was supported by the Medical Research Council of Canada. I. A. M. de Lannoy was a recipient of the Ontario Graduate Scholarship from the Ontario Ministry of Health; K. S. Pang was a recipient of the Faculty Development Award, Medical Research Council.
Rights and permissions
About this article
Cite this article
de Lannoy, I.A.M., Pang, K.S. Combined recirculation of the rat liver and kidney: Studies with enalapril and enalaprilat. Journal of Pharmacokinetics and Biopharmaceutics 21, 423–456 (1993). https://doi.org/10.1007/BF01061690
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01061690