Summary
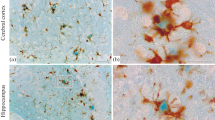
We studied the nature of diffuse type of senile plaques (SP) in the brains of six autopsied subjects with Alzheimer-type dementia (ATD). The densities of SP in the entorhinal cortex were evaluated using serial sections stained by four different methods. Compared with β protein immunostaining (100% as a reference), the modified Bielschowsky stain (103%) and the periodic acid-methenamine silver (PAM) stain (109%) labeled similar numbers of SP, whereas the Bodian stain labeled only a minor proportion (42%) of these. The vast majority of Bodian-negative plaques were diffuse plaques, which were seen as ill-defined areas of fine fibrillar material after β protein immunostain with formic acid pretreatment, modified Bielschowsky stain, and PAM stain. They were not stained by Congo red or periodic acid-Schiff stains. Double staining using Bodian and β protein methods demonstrated that diffuse plaques were free of swollen neurites. Argyrophilia of the diffuse plaques shown by the modified Bielschowsky and PAM stains, became undetectable when sections were pretreated with formic acid. Such treatment made the diffuse plaques immunoreactive to β protein antiserum, suggesting that diffuse plaques consisted mainly of amyloid, but not neuritic components. The diffuse plaques were distributed in various cortical areas and in the amygdala, and comprised a considerable population of the SP in the ATD brains.
Similar content being viewed by others
References
Allsop D, Landon M, Kidd M, Lowe JS, Reynolds GP, Gardner A (1986) Monoclonal antibodies raised against a subsequence of senile plaque core prtoein react with plaque cores, plaque periphery and cerebrovascular amyloid in Alzheimer's disease. Neurosci Lett 68:252–256
Blessed G, Tomlinson BE, Roth M (1968) The association between quantitative measures of dementia and of senile changes in the cerebral grey matter of elderly subjects. Br J Psychiatry 114:797–811
Blocq P, Marinesco G (1892) Sur les lésions et la pathogénie de l'épilepsie dite essentielle. Sem Med 12:445–446
Bodian D (1936) A new method for staining nerve fibers and nerve endings in mounted paraffin sections. Anat Rec 65:89–97
Braunmühl A von (1957) Alterserkrankungen des Zentral-nervensystems. Senile Involution. Alzheimer'sche Krankheit. In: Lubarsch O, Henke F, Rössel R (eds) Handbuch der Speziellen Pathologischen Anatomie und Histologie XIII, vol 1A. Erkrankungen des Zentralnervensystems. Springer, Berlin Göttingen Heidelberg, pp 337–539
Fischer O (1910) Die presbyophrene Demenz, deren anatomische Grundlage und klinische Abgrenzung. Z Gesamte Neurol Psychiatr 3:371–471
Gibson PH (1983) Form and distribution of senile plaques seen in silver impregnated sections in the brains of intellectually normal elderly people and people with Alzheimer-type dementia. Neuropathol Appl Neurobiol 9:379–389
Glenner GG, Wong CE (1984) Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890
Hirai S (1983) Dementia: pathophysiology. Proceedings of the 2nd Regional Congress on International Association of Gerontology, Singapore. Australian Association of Gerontology, Sydney, pp 31–34
Kang J, Lemaire H-G, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K-H, Multhaup G, Bayreuther K, Müller-Hill B (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736
Kitamoto T, Ogomori K, Tateishi J, Prusiner SB (1987) Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloidosis. Lab Invest 57:230–236
Luna LG (1968) Manual of histologic staining methods of the Armed Forces Institute of Pathology, 3rd edn. McGraw-Hill, New York, pp 97–99
Makifuchi T, Watabe K, Takahashi H, Ikuta F (1986) Amyloid in senile plaque stained by periodic acid-silver methenamine. Abstracts of the 10th International Congress on Neuropathology. Stockholm Convention Bureau, Stockholm, p 417
Makifuchi T, Takahashi H, Ikuta F (1987) Amyloid in primitive senile plaque: serial PAM electron microscopic study. Annual report of Slow Virus Infection Research Committee, 1986. Ministry of Health and Welfare of Japan, Tokyo, pp 110–113
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82:4245–4249
Miyakawa T, Katsurgi S, Watanabe K, Shimoji A, Ikeuchi Y (1986) Ultrastructural studies of amyloid fibrils and senile plaques in human brain. Acta Neuropathol (Berl) 70:202–208
Morimatsu M, Hirai S, Muramatsu A, Yoshikawa M (1975) Senile degenerative brain lesions and dementia. J Am Geriatr Soc 23:390–406
Pardrige WM, Vinters HV, Miller BL, Tourtellotte WW, Eisenberg JB, Yang J (1987) High molecular weight Alzheimer's disease amyloid peptide immunoreactivity in human serum and CSF is an immunoglobulin in G. Biochem Biophys Res Commun 145:241–248
Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC (1987) Conservations of brain amyloid protein in aged mammals and humans with Alzheimer's disease. Science 235:873–877
Tomlinson BE, Corsellis JAN (1984) Aging and the dementias. In: Adams JH, Corsellis JAN, Duchen LW (eds) Greenfield's neuropathology, 4th edn. Edward Arnold, London, pp 951–1025
Tsujiyama Y (1934) Contribution à l'étude histopathologique des plaques seniles. Keio J Med 14:1529–1541
Ulrich J (1985) Alzheimer changes in nondemented patients younger than sixty-five: possible early stages of Alzheimer's disease and senile dementia of Alzheimer type. Ann Neurol 17:273–277
Uyematsu S (1923) On the pathology of senile psychosis. J Nerv Ment Dis 57:1–25
Uyematsu S (1923) On the pathology of senile psychosis. J Nerv Ment Dis 131–156
Uyematsu S (1923) On the pathology of senile psychosis. J Nerv Ment Dis 237–260
Wisniewsky HM, Terry RD (1973) Reexamination of the pathogenesis of the senile plaque. Prog Neuropathol 2:1–26
Wong CW, Quaranta V, Glenner GG (1985) Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci USA 82:8729–8732
Yamaguchi H, Morimatsu M, Okamoto K, Hirai S (1987) Senile dementia of Alzheimer type: relationship between types of senile plaques and dementia. Clin Neurol (Tokyo) 27:617–621
Yamaguchi H, Hirai S, Morimatsu M, Shooji M, Ihara Y (1988) A variety of cerebral amyloid deposits in the brains of the Alzheimer-type dementia demonstrated by β protein immunostaining. Acta Neuropathol 76:541–549
Yamamoto T, Hirano A (1986) A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer's neurofibrillary tangles. Neuropathol Appl Neurobiol 12:3–9
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamaguchi, H., Hirai, S., Morimatsu, M. et al. Diffuse type of senile plaques in the brains of Alzheimer-type dementia. Acta Neuropathol 77, 113–119 (1988). https://doi.org/10.1007/BF00687420
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00687420