Abstract
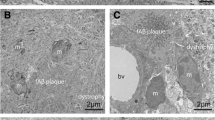
Extracellular accumulation of Aβ peptides and intracellular aggregation of hyperphosphorylated tau proteins are the two hallmark lesions of Alzheimer disease (AD). The senile plaque is made of a core of extracellular Aβ surrounded by phospho-tau positive neurites. It includes multiple components such as axons, synapses, glial fibers and microglia. To visualize the relationships of those elements, an original technique was developed, based on the dilation of interstitial water during freezing. Samples of neocortex, hippocampus and striatum were taken from formalin-fixed brains (one control case; three cases with severe Alzheimer disease). The samples were subjected to various numbers of freezing/thawing cycles (from 0 to 320) with an automated system we devised. The samples were embedded in paraffin, cut and stained with haematoxylin-eosin or immunostained against Aβ, phospho-tau, and antigens enriched in axons, synapses, macrophages or astrocytes. Microcryodissection induced the dissociation of tissue components, especially in the grey matter where the neuropil formed an oriented “mesh”. The size of the empty spaces separating the fiber bundles and cells increased with the number of cycles. The amyloid core of the senile plaque separated from its neuritic crown at around 300 freezing/thawing cycles. The dissected core remained associated with macrophages containing Aβ in their cytoplasm. Phospho-tau positive axons were distinctly seen projecting from the neuritic crown to the isolated amyloid core, where they ended in large synapses. The microcryodissection showed astrocytic processes stuck directly to the core. The original method we developed—microcryodissection—helped understanding how histological components were assembled in the tissue.





Similar content being viewed by others
References
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Del Tredici K (2014) Are cases with tau pathology occurring in the absence of Aβ deposits part of the AD-related pathological process? Acta Neuropathol 128:767–772. doi:10.1007/s00401-014-1356-1
Duyckaerts C, Delatour B, Potier M-C (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118:5–36. doi:10.1007/s00401-009-0532-1
Eisele YS, Duyckaerts C (2016) Propagation of Aβ pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 131:5–25. doi:10.1007/s00401-015-1516-y
Frackowiak J, Wisniewski HM, Wegiel J et al (1992) Ultrastructure of the microglia that phagocytes amyloid and the microglia that produces beta-amyloid fibrils. Acta Neuropathol 84:223–225
Gonatas NK, Anderson W, Evangelista I (1967) The contribution of altered synapses in the senile plaque: an electron microscopic study in Alzheimer’s dementia. J Neuropathol Exp Neurol 26:25–39
Ludwig E, Klingler J (1956) Atlas cerebri humani. S. Karger, Basel
Schmidt ML, Lee VM, Trojanowski JQ (1991) Comparative epitope analysis of neuronal cytoskeletal proteins in Alzheimer’s disease senile plaque, neurites and neuropil threads. Lab Invest 64:352–357
Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Weibel ER (1979) Stereological methods. Practical methods for biological morphometry. Academic Press, London
Acknowledgements
Electron microscopy was performed at the imaging platform of Pitié-Salpêtrière (PICPS) located at ICM. Alice Lebois helped to develop the freezing/thawing device. The samples were obtained from the Brain Bank Neuro-CEB (BRIF number 0033-00011), funded by the patients’ associations France Parkinson, ARSEP, and “Connaître les Syndromes Cérébelleux” and by LECMA Vaincre Alzheimer, to which we express our gratitude. The Brain Bank is hosted by the Biological Resource Platform of Pitié-Salpêtrière Hospital, APHP.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thierry, M., Marty, S., Boluda, S. et al. Alzheimer’s senile plaque as shown by microcryodissection, a new technique for dissociating tissue structures. J Neural Transm 124, 685–694 (2017). https://doi.org/10.1007/s00702-017-1718-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-017-1718-7