Abstract
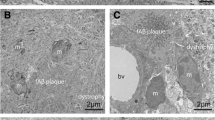
The close spatial relationship of glial cells (astroglia and microglia) to amyloid plaques is one of the histopathological features of Alzheimer’s disease. The work aimed to develop a simple and informative method for the simultaneous detection of amyloid plaques and glial cells. The suggested method is based on a combination of histochemical staining for amyloid and non-fluorescent immunohistochemical staining of glial cells. The cerebral cortex samples of aged people (n = 8) and brain samples from transgenic 5×FAD mice (n = 6) were the material for this study. The proposed methodological approach involves immunohistochemical labelling of microglia or astroglia followed by Alcian Blue staining. The developed protocol is simple and well reproducible. It is suitable for identifying the association of glial cells with amyloid plaques. It was noted that immunohistochemistry for Iba1 (ionized calcium binding adaptor molecule 1) and for GFAP (glial fibrillary acidic protein) allows the most effective identification of microgliocytes and astrocytes, respectively. It is also suitable for assessing the functional status of these cells. Amyloid plaques are intensely stained with Alcian Blue and are well detected. In comparison with double immunofluorescence staining technique for simultaneous detection of glial cells and amyloid plaques, the developed method is simple to implement and requires only a light microscope, which makes it accessible to most laboratories.




Similar content being viewed by others
REFERENCES
Acosta, C., Anderson, H.D., and Anderson, C.M., Astrocyte dysfunction in Alzheimer disease, J. Neurosci. Res., 2017, vol. 95, p. 2430. https://doi.org/10.1002/jnr.24075
Alekseeva, O.S., Kirik, O.V., Gilerovich, E.G., and Korzhevskii, D.E., Microglia of the brain: origin, structure, functions, J. Evol. Biochem. Physiol., 2019, vol. 55, p. 257. https://doi.org/10.1134/S002209301904001X
Boom, A., Pochet, R., Authelet, M., Pradier, L., Borghgraef, P., Van Leuven, F., Heizmann, C.W., and Brion, J.P., Astrocytic calcium/zinc binding protein S100A6 over expression in Alzheimer’s disease and in PS1/APP transgenic mice models, Biochim. Biophys. Acta, Mol. Cell Res., 2004, vol. 1742, p. 161. https://doi.org/10.1016/j.bbamcr.2004.09.011
Brozzi, F., Arcuri, C., Giambanco, I., and Donato, R., S100B protein regulates astrocyte shape and migration via interaction with Src kinase, J. Biol. Chem., 2009, vol. 284, p. 8797. https://doi.org/10.1074/jbc.M805897200
Chumasov, E.I., Korzhevskii, D.E., Petrova, E.S., Kuznetsova, N.N., and Sapronov, N.S., Glial reaction of the subventricular zone of the telencephalon of the rat brain on modeling of Alzheimer’s disease, Neurosci. Behav. Physiol., 2012, vol. 42, p. 67. https://doi.org/10.1007/s11055-011-9535-1
Condello, C., Yuan, P., Schain, A., and Grutzendler, J., Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques, Nat. Commun., 2015, vol. 6, p. 6176. https://doi.org/10.1038/ncomms7176
Donato, R., Intracellular and extracellular roles of S100 proteins, Microsc. Res. Techn., 2003, vol. 60, p. 540. https://doi.org/10.1002/jemt.10296
Dossi, E., Vasile, F., and Rouach, N., Human astrocytes in the diseased brain, Brain Res. Bull., 2018, vol. 136, p. 139. https://doi.org/10.1016/j.brainresbull.2017.02.001
Ferrer, I., Diversity of astroglial responses across human neurodegenerative disorders and brain aging, Brain Pathol., 2017, vol. 27, p. 645. https://doi.org/10.1111/bpa.12538
Fong, A.Y., Stornetta, R.L., Foley, C.M., and Potts, J.T., Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: subregional distribution in the nucleus tractus solitarius, J. Comp. Neurol., 2005, vol. 493, p. 274. https://doi.org/10.1002/cne.20758
Garaschuk, O. and Verkhratsky, A., GABAergic astrocytes in Alzheimer’s disease, Aging, 2019, vol. 11, p. 1602. https://doi.org/10.18632/aging.101870
Garwood, C.J., Ratcliffe, L.E., Simpson, J.E., Heath, P.R., Ince, P.G., and Wharton, S.B., Review: astrocytes in Alzheimer’s disease and other age-associated dementias: a supporting player with a central role, Neuropathol. Appl. Neurobiol., 2017, vol. 43, p. 281. https://doi.org/10.1111/nan.12338
Guénette, S.Y., Astrocytes: a cellular player in Aβ clearance and degradation, Trends Mol. Med., 2003, vol. 9, p. 279. https://doi.org/10.1016/S1471-4914(03)00112-6
Guselnikova, V., Antipova, M.V., Fedorova, E.A., Safray, A.E., Rukavishnikova, A.A., Mikhailova, E.V., and Korzhevskii, D.E., Distinctive features of histochemical and immunohistochemical techniques for amyloid plaque detection in the human cerebral cortex, J. Anat. Histopathol., 2019, vol. 8, p. 91. https://doi.org/10.18499/2225-7357-2019-8-2-91-99
Habib, N., McCabe, C., Medina, S., Varshavsky, M., Kitsberg, D., Dvir-Szternfeld, R., Green, G., Dionne, D., Nguyen, L., Marshall, J.L., Chen, F., Zhang, F., Kaplan, T., Regev, A., and Schwartz, M., Disease-associated astrocytes in Alzheimer’s disease and aging, Nat. Neurosci., 2020, vol. 23, p. 701. https://doi.org/10.1038/s41593-020-0624-8
Halle, A., Hornung, V., Petzold, G.C., Stewart, C.R., Monks, B.G., Reinheckel, T., Fitzgerald, K.A., Latz, E., Moore, K.J., and Golenbock, D.T., The NALP3 inflammasome is involved in the innate immune response to amyloid-β, Nat. Immunol., 2008, vol. 9, p. 857. https://doi.org/10.1038/ni.1636
Hansen, D.V, Hanson, J.E., and Sheng, M., Microglia in Alzheimer’s disease, J. Cell Biol., 2018, vol. 217, p. 459. https://doi.org/10.1083/jcb.201709069
Hopperton, K.E., Mohammad, D., Trépanier, M.O., Giuliano, V., and Bazinet, R.P., Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: a systematic review, Mol. Psychiatry, 2018, vol. 23, p. 177. https://doi.org/10.1083/jcb.201709069
Jo, S., Yarishkin, O., Hwang, Y.J., Chun, Y.E., Park, M., Woo, D.H., Bae, J.Y., Kim, T., Lee, J., Chun, H., Park, H.J., Lee, D.Y., Hong, J., Kim, H.Y., Oh, S.J., and et, al., GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease, Nat. Med., 2014, vol. 20, p. 886. https://doi.org/10.1038/nm.3639
Kamphuis, W., Mamber, C., Moeton, M., Kooijman, L., Sluijs, J.A., Jansen, A.H.P., Verveer, M., de Groot, L.R., Smith, V.D., Rangarajan, S., Rodríguez, J.J., Orre, M., and Hol, E.M., GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease, PLoS One, 2012, vol. 7, article ID e42823. https://doi.org/10.1371/journal.pone.0042823
Katsouri, L., Birch, A.M., Renziehausen, A.W.J., Zach, C., Aman, Y., Steeds, H., Bonsu, A., Pal-mer, E.O.C., Mirzaei, N., Ries, M., and Sastre, M., Ablation of reactive astrocytes exacerbates disease pathology in a model of Alzheimer’s disease, Glia, 2020, 2020, vol. 68, p. 1017. https://doi.org/10.1002/glia.23759
Kelly, P., Hudry, E., Hou, S.S., and Bacskai, B.J., In vivo two photon imaging of astrocytic structure and function in Alzheimer’s disease, Front. Aging Neurosci., 2018, vol. 10, p. 219. https://doi.org/10.3389/fnagi.2018.00219
Keren-Shaul, H., Spinrad, A., Weiner, A., Matcovitch-Natan, O., Dvir-Szternfeld, R., Ulland, T.K., David, E., Baruch, K., Lara-Astaiso, D., Toth, B., Itzkovitz, S., Colonna, M., Schwartz, M., and Amit, I., A unique microglia type associated with restricting development of Alzheimer’s disease, Cell, 2017, vol. 169, p. 1276. https://doi.org/10.1016/j.cell.2017.05.018
Kirik, O.V., Sukhorukova, E.G., and Korzhevskii, D.E., Calcium-binding protein Iba-1/AIF-1 in rat brain cells, Neurosci. Behav. Physiol., 2011, vol. 41, p. 149. https://doi.org/10.1007/s11055-011-9391-z
Korzhevskii, D.E., Kirik, O.V., Petrova, E.S., Karpenko, M.N., Grigoriev, I.P., Sukhorukova, E.G., Kolos, E.A., and Gilyarov, A.V., Teoreticheskie osnovy i prakticheskoe primenenie metodov immunogistokhimii (Theoretical Foundations and Practical Application of Methods of Immunohistochemistry), Korzhevsky, D.E., Ed., St. Petersburg: SpetsLit, 2014, 2nd ed. (rev., add.).
Korzhevskii, D.E., Sukhorukova, E.G., Kirik, O.V., and Grigorev, I.P., Immunohistochemical demonstration of specific antigens in the human brain fixed in zinc-ethanol-formaldehyde, Eur. J. Histochem., 2015, vol. 59, p. 5. https://doi.org/10.4081/ejh.2015.2530
Korzhevskii, D.E., Grigor’ev, I.P., Gusel’nikova, V.V., Kolos, E.A., Petrova, E.S., Kirik, O.V., Sufieva, D.A., Razenkova, V.A., Antipova, M.V., and Chernysh, M.V., Immunohistochemical markers for neurobiology, Med. Acad. J., 2019, vol. 19, p. 7. https://doi.org/10.17816/MAJ16548
LaFerla, F.M. and Green, K.N., Animal models of Alzheimer disease, Cold Spring Harbor Persp. Med., 2012, vol. 2, article ID a006320. https://doi.org/10.1101/cshperspect.a006320
Li, K.-Y., Gong, P., Li, J., Xu, N., and Qin, S., Morphological and molecular alterations of reactive astrocytes without proliferation in cerebral cortex of an APP/PS1 transgenic mouse model and Alzheimer’s patients, Glia, 2020, vol. 68, p. 2361. https://doi.org/10.1002/glia.23845
Mucke, L., Alzheimer’s disease, Nature, 2009, vol. 461, p. 895. https://doi.org/10.1038/461895a
Nagele, R.G., D’Andrea, M.R., Lee, H., Venkataraman, V., and Wang, H.-Y., Astrocytes accumulate aβ42 and give rise to astrocytic amyloid plaques in Alzheimer disease Brains, Brain Res., 2003, vol. 971, p. 197. https://doi.org/10.1016/S0006-8993(03)02361-8
Nicoll, J.A.R. and Weller, R.O., A new role for astrocytes: β-amyloid homeostasis and degradation, Trends Mol. Med., 2003, vol. 9, p. 281. https://doi.org/10.1016/S1471-4914(03)00109-6
Nosova, O.I., Sufieva, D.A., and Korzhevskii, D.E., An astrocyte structural organization analysis based on fluorescent microscopy with 2D and 3D quantitative approaches, Cell Tissue Biol., 2021, vol. 15, p. 273. https://doi.org/, 2021, vol. 101134/S1990519X21030081.
Oakley, H., Cole, S.L., Logan, S., Maus, E., Shao, P., Craft, J., Guillozet-Bongaarts, A., Ohno, M., Disterhoft, J., Van Eldik, L., Berry, R., and Vassar, R., Intraneuronal beta-amyloid aggregates, meurodegeneration, and meuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation, J. Neurosci., 2006, vol. 26, p. 10129. https://doi.org/10.1523/JNEUROSCI.1202-06.2006
Olabarria, M., Noristani, H.N., Verkhratsky, A., and Rodríguez, J.J., Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease, Glia, 2010, vol. 58, p. 831. https://doi.org/10.1002/glia.20967
Olabarria, M., Noristani, H.N., Verkhratsky, A., and Rodríguez, J.J., Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: mechanism for deficient glutamatergic transmission?, Mol. Neurodegeneration, 2011, vol. 6, p. 55. https://doi.org/10.1186/1750-1326-6-55
Orellana, J.A., Shoji, K.F., Abudara, V., Ezan, P., Amigou, E., Saez, P.J., Jiang, J.X., Naus, C.C., Saez, J.C., and Giaume, C., Amyloid-induced death in neurons involves glial and neuronal hemichannels, J. Neurosci., 2011, vol. 31, p. 4962. https://doi.org/10.1523/JNEUROSCI.6417-10.2011
Poliakova, A.A., Semernin, E.N., Sitnikova, M.Y., Avagyan, K.L., Grozov, R.V., Pyko, S.A., Krutikov, A.N., Davydova, V.G., Khmelnitskaya, K.A., Shavloskii, M.M., Korzhevskii, D.E., and Gudkova, A.Y., Transthyretin amyloidosis in a cohort of old and very old patients with chronic heart failure, Kardiologiya, 2018, vol. 58, p. 12. https://doi.org/10.18087/cardio.2390
Pomerance, A., Slavin, G., and McWatt, J., Experience with the sodium sulphate-alcian blue stain for amyloid in cardiac pathology, J. Clin. Pathol., 1976, vol. 29, p. 22. https://doi.org/10.1136/jcp.29.1.22
Razenkova, V.A. and Korzhevskii, D.E., GABAergic axosomatic synapses of rat brain cortex, Cell Tissue Biol., 2021, vol. 15, p. 267. https://doi.org/10.1134/S1990519X21030093
Rodríguez-Arellano, J.J., Parpura, V., Zorec, R., and Verkhratsky, A., Astrocytes in physiological aging and Alzheimer’s disease, Neuroscience, 2016, vol. 323, p. 170. https://doi.org/10.1016/j.neuroscience.2015.01.007
Simpson, J.E., Ince, P.G., Lace, G., Forster, G., Shaw, P.J., Matthews, F., Savva, G., Brayne, C., and Wharton, S.B., Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain, Neurobiol. Aging, 2010, vol. 31, p. 578. https://doi.org/10.1016/j.neurobiolaging.2008.05.015
Snow, P., Patel, N., Harrelson, A., and Goodman, C., Neural-specific carbohydrate moiety shared by many surface glycoproteins in drosophila and grasshopper embryos, J. Neurosci., 1987, vol. 7, p. 4137. https://doi.org/10.1523/JNEUROSCI.07-12-04137.1987
Snow, A.D., Sekiguchi, R.T., Nochlin, D., Kalaria, R.N., and Kimata, K., Heparan sulfate proteoglycan in diffuse plaques of hippocampus but not of cerebellum in Alzheimer’s disease brain, Am. J. Pathol., 1994, vol. 144, p. 337. http://www.ncbi.nlm.nih.gov/pubmed/8311117.
Steiner, J., Bernstein, H.-G., Bielau, H., Berndt, A., Brisch, R., Mawrin, C., Keilhoff, G., and Bogerts, B., Evidence for a wide extra-astrocytic distribution of S100B in human brain, BMC Neurosci., 2007, vol. 8, p. 2. https://doi.org/10.1186/1471-2202-8-2
Stepanichev, M.Y., Zdobnova, I.M., Zarubenko, I.I., Lazareva, N.A., and Gulyaeva, N.V., Studies of the effects of central administration of β-amyloid peptide (25–35): pathomorphological changes in the hippocampus and impairment of spatial memory, Neurosci. Behav. Physiol., 2006, vol. 36, p. 101. https://doi.org/10.1007/s11055-005-0167-1
Tzioras, M., Davies, C., Newman, A., Jackson, R., and Spires-Jones, T., APOE at the interface of inflammation, neurodegeneration and pathological protein spread in Alzheimer’s disease, Neuropathol. Appl. Neurobiol., 2019, vol. 45, p. 327. https://doi.org/10.1111/nan.12529
Verkhratsky, A., Olabarria, M., Noristani, H.N., Yeh, C., and Rodriguez, J.J., Astrocytes in Alzheimer’s disease, Neurotherapeutics, 2010, vol. 7, p. 399. https://doi.org/10.1016/j.nurt.2010.05.017
Verkhratsky, A., Zorec, R., Rodriguez, J.J., and Parpura, V., Pathobiology of neurodegeneration: the role for astroglia, Opera Med. Physiol., 2016, vol. 1, p. 13. https://doi.org/10.20388/OMP2016.001.0019
Verkhratsky, A., Rodrigues, J.J., Pivoriunas, A., Zorec, R., and Semyanov, A., Astroglial atrophy in Alzheimer’s disease, Pflügers Arch., 2019, vol. 471, p. 1247. https://doi.org/10.1007/s00424-019-02310-2
Wu, Z., Guo, Z., Gearing, M., and Chen, G., Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s disease model, Nat. Commun., 2014, vol. 5, p. 4159. https://doi.org/10.1038/ncomms5159
Yeh, C.-Y., Vadhwana, B., Verkhratsky, A., and Rodríguez, J.J., Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer’s disease, ASN Neuro, 2011, vol. 3, p. 272. https://doi.org/10.1042/AN20110025
ACKNOWLEDGMENTS
The work was carried out the state assignment of the Institute of Experimental Medicine.
Funding
The work of V.V. Guselnikova was supported by a grant from the President of the Russian Federation to support young russian scientists (MK-560.2020.7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that there is no conflict of interests
Statement on the welfare of animals. All applicable international and national principles of humane treatment of animals were observed. All the experiments were approved by the Local Ethics Committee of Institute of Experimental Medicine (protocol no. 3/18 dated November 22, 2018).
Additional information
Abbreviations: AD – Alzheimer’s disease; GFAP – glial fibrillar acidic protein.
Rights and permissions
About this article
Cite this article
Nosova, O.I., Guselnikova, V.V. & Korzhevskii, D.E. Light Microscopy Approach for Simultaneous Identification of Glial Cells and Amyloid Plaques. Cell Tiss. Biol. 16, 140–149 (2022). https://doi.org/10.1134/S1990519X22020080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X22020080