Summary
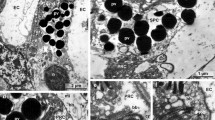
The photoreceptor of Amaroucium constellatum consists of three parts: lens cells, pigment cup, and retinal cells. The three lens cells lie just beneath the epidermis and extend ventrally into the sensory vesicle. A large part of their volume is occupied by a lens vesicle, a region bounded by mitochondria. The function of the lens cells is unclear. The pigment cup, a hollow hemisphere formed by the pigment cell, acts as a light shield, presumably allowing the tadpole to respond directionally to light. No unusual morphological features of this cell have been noticed. Each of the seven to ten photoreceptor cells sends a process through the pigment cup towards the lens cells. A cilium at the tip of each process gives rise to membranous lamellae which are presumably photosensitive. The base of each photoreceptor cell seems to send processes to the cerebral ganglion. No synapses have been seen at the base of the photoreceptor cells. The cerebral ganglion consists of a cellular cortex surrounding a medullary region of neuropil. Two types of synapses, presumably chemical and electrical, are evident. Four classes of vesicles are found in these structures.
Similar content being viewed by others
References
Barnes, S. N.: The fine structure of the photoreceptor of the ascidian, Amaroucium constellalatum. Biol. Bull. 137, 392 (1969).
Bell, A. L., Barnes, S. N., Anderson, K. L.: A fixation technique for electron microscopy which provides uniformly good preservation of the tissues of a variety of marine invertebrates. Biol. Bull. 137, 393 (1969).
Bennett, H. S., Luft, J. H.: S-collidine as a basis for buffering fixatives. J. biophys. biochem. Cytol. 6, 113–114 (1959).
Bennett, M. V. L., Aljure, E., Nakajima, Y., Pappas, G. D.: Electrotonic junctions between teleost neurons: electrophysiology and ultrastructure. Science 141, 262–263 (1963).
Cohen, A. I.: New evidence supporting the linkage to extracellular space of outer segment saccules of frog cones but not rods. J. Cell Biol. 37, 424–444 (1968).
Costello, D. P., Davidson, M. E., Eggers, A., Fox, M. H., Henley, C.: Methods for obtaining and handling marine eggs and embryos. Woods Hole, Mass.: Marine Biological Laboratory 1957.
Deck, J. D., Hay, E. D., Revel, J. P.: Fine structure and origin of the tunic of Perophora viridis. J. Morph. 120, 267–280 (1966).
De Robertis, E.: Morphogenesis of retinal rods. J. biophys. biochem. Cytol. 2, 209–220 (1956).
—: Fine structure of synapses in the C.N.S. Proc. 4th Intern. Cong. Neuro. Path. 2, 35–38 (1962).
Dilly, P. N.: Electron microscope observations of the receptors in the sensory vesicle of the ascidian tadpole. Nature (Lond.) 191, 786–787 (1961).
—: Studies on the receptors in the cerebral vesicle of the ascidian tadpole. 2. The ocellus. Quart. J. micr. Sci. 105, 13–20 (1964).
—: Synapses in the cerebral ganglion of the adult Ciona intestinalis. Z. Zellforsch. 93, 142–150 (1969).
Dowling, J. E.: Synaptic organization of the frog retina: an electron microscopic analysis comparing the retinas of frogs and primates. Proc. roy. Soc. B, 170, 205–228 (1968).
Eakin, R. M.: Lines of evolution of photoreceptors. In: General physiology of cell specialization, eds. Mazia, D., and A. Tyler, p. 393–425. New York: McGraw-Hill 1963.
—: Evolution of photoreceptors. Cold Spr. Harb. Symp. quant. Biol. 30, 363–370 (1965).
—: Evolution of photoreceptors. In: Evolutionary biology, eds. Dobzhansky, T., M. K. Hecht, and W. C. Steere, vol. 2, p. 194–242. New York: Appleton-Century Crofts 1968.
Furshpan, E. J.: “Electrical transmission” at an excitatory synapse in a vertebrate brain. Science 144, 878–880 (1964).
Graves, C.: Amaroucium constellatum (Verrill) II. The structure and organization of the tadpole larva. J. Morph. 36, 71–101 (1921).
Gray, E. G.: Problems of interpreting the fine structure of vertebrate and invertebrate synapses. Int. Rev. gen. exp. Zool. 2, 139–170 (1966).
Kandel, E. R., Kupfermann, I.: The functional organization of invertebrate ganglia. Ann. Rev. Physiol. 32, 193–258 (1970).
Luft, J.: Improvements in epoxy embedding methods. J. biophys. biochem. Cytol. 9, 409–414 (1961).
Maser, M. D., Powell, T. E., Philpott, C. W.: Relationships among pH, osmolality, and concentration of fixative solutions. Stain Technol. 42, 175–182 (1967).
Mast, S. O.: Reactions to light in the ascidians, Amaroucium constellatum and Amaroucium pellucidum with special reference to photic orientation. J. exp. Zool. 34, 149–187 (1921).
Palade, G. E.: A study of fixation for electron microscopy. J. exp. Med. 95, 285–298 (1952).
Revel, J. P., Karnovsky, M. J.: Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J. Cell Biol. 33, C7-C12 (1967).
- Olson, W., Karnovsky, M. J.: A twenty-angstrom gap junction with a hexagonal array of subunits in smooth muscle. J. Cell Biol. 35, 112A (1967).
Reynolds, E.: The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 17, 208–212 (1963).
Robertson, J. D.: Unit membranes: A review with recent new studies of experimental alterations and a new subunit structure in synaptic membranes. Proceedings of the 22nd Symposium of the Society for the Study of Development and Growth (M. Locke, ed.), p. 1–8. New York: Academic Press 1964.
Sabatini, D. E., Bensch, K., Barrnett, R. J.: Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol. 17, 19–58 (1963).
Scott, F. M. Sister: The developmental history of Amaroecium constellatum. III. Metamorphosis. Biol. Bull. 103, 226–241 (1952).
Sjöstrand, F. S.: The ultrastructure of the retinal receptors of the vertebrate eye. Ergebn. Biol. 21, 128–160 (1959).
Watson, M. L.: Staining of tissue sections for electron microscopy with heavy metals. J. biophys. biochem. Cytol. 4, 475–478 (1958).
Author information
Authors and Affiliations
Additional information
This investigation was supported in part by Training Grant number GMO 1981 from the National Institutes of Health, by Health Science Achievement Award number RR06084, and Grant number EY00443 (to Dr. A. L. Bell) from the National Institutes of Health.
I wish to thank Dr. Allen L. Bell for his invaluable assistance and encouragement during this investigation.
Rights and permissions
About this article
Cite this article
Barnes, S.N. Fine structure of the photoreceptor and cerebral ganglion of the tadpole larva of Amaroucium constellatum (Verrill) (Subphylum: Urochordata; Class: Ascidiacea). Z. Zellforsch. 117, 1–16 (1971). https://doi.org/10.1007/BF00331097
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00331097