Abstract
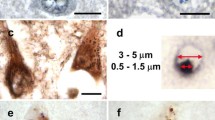
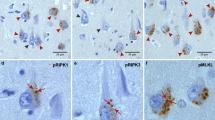
Ubiquitin-immunoreactive dystrophic neurites in the CA2/3 region of the hippocampus are characteristic of diffuse Lewy body disease (DLBD). The origin of dystrophic CA2/3 neurites is unknown, but their extent correlates with the number of cortical Lewy bodies (LBs). To examine the molecular composition of these lesions, hippocampal sections were obtained at postmortem from cases of DLBD, Parkinson's disease and Alzheimer's disease. The tissue samples were fixed in a variety of fixatives and immunostained with antibodies to ubiquitin, ubiquitin C-terminal hydrolase (PGP9.5), neurofilament protein subunits, tau protein, paired helical filaments and tyrosine hydroxylase (TH). In addition to being ubiquitin positive, both cortical LBs and CA2/3 dystrophic neurites were positive with a neurofilament monoclonal antibody (RM032) and PGP9.5; however, fewer lesions were detected with these antibodies compared to ubiquitin immunocyto-chemistry. The dystrophic CA2/3 neurites were not stained with antibodies to tau proteins, paired helical filaments or TH. Absence of TH immunoreactivity suggests that CA2/3 neuritic processes are not derived from brain stem dopaminergic afferents to the hippocampus. Since CA2/3 neurites are immunologically similar to cortical LB, the pathogenesis of these lesions may be similar. Characterization of dystrophic CA2/3 neurites and cortical LBs may clarify how these lesions contribute to the emergence of dementia in DLBD.
Similar content being viewed by others
References
Arai H, Lee VM-Y, Hill WD, Greenberg BD, Trojanowski JQ (1992) LBs contain beta-amyloid precursor proteins of Alzheimer's disease. Brain Res 585: 386–390
Bancher C, Lassmann H, Budka H, Jellinger K, Grundke-Iqbal I, Iqbal K, Wiche G, Seitelberger F, Wisniewski HM (1989) An antigenic profile of LBs: immunocytochemical indication for protein phosphorylation and ubiquitination. J Neuropathol Exp Neurol 1989. 48: 81–93
Bischoff S (1986) Mesohippocampal dopamine system: characterization, functional and clinical implications. In: Isaacson AL, Pribram KH (eds) The hippocampus, vol. 3 Plenum Press, New York, pp 1–32
Burkhardt CR, Filley CM, Kleinschmidt-DeMasters BK, de la Monte S, Norenberg MD, Schneck SA (1988) Diffuse Lewy body disease and progressive dementia. Neurology 38: 1520–1528
Byrne EJ, Lennox G, Lowe J, Godwin-Austen RB (1989) Diffuse Lewy body disease: clinical features in 15 cases. J Neurol Neurosurg Psychiatry 52: 709–717
Crystal H, Dickson DW, Lizardi JE, Davies P, Wolfson LI (1990) Antemortem diagnosis of diffuse Lewy body disease. Neurology 40: 1523–1528
De Keyser J, Herregodts P, Eliinger G (1990) The mesoneocortical dopamine neuron system. Neurology 40: 1660–1662
Dickson DW, Davies P, Mayeux R, Crystal HA, Thompson D, Goldman JE (1987) Diffuse Lewy body disease: neuropathological and biochemical studies of six patients. Acta Neuropathol (Berl) 75: 8–15
Dickson DW, Crystal H, Mattiace LA, Kress Y, Schwagerl A, Davies P, Yen S-H (1989) Diffuse Lewy body disease: light and electron microscopic immunocytochemistry of senile plaques. Acta Neuropathol 78: 572–584
Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, Kress Y, Yen S-H (1991) Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer's disease: light and electron microscopic immunocytochemistry of CA23 neurites specific to DLBD. Neurology 41: 1402–1409
Dickson DW, Wu E, Crystal HA, Mattiace LA, Yen S-H, Davies P (1992) Alzheimer and age-related pathology in diffuse Lewy body disease. In: Boller F, Forcette F, Khachaturian Z, Poncet M, Christen Y (eds) Heterogeneity of Alzheimer's disease. Springer-Verlag Berlin Heidelberg New York Tokyo, pp 168–186
Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen S-H, Aronson MK (1992) Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging 13: 179–189
Eggertson DE, Sima AAF (1986) Dementia with cerebral LBs: a mesocortical dopaminergic defect? Arch Neurol 43: 524–527
Forno LS, Sternberger LA, Sternberger NH, Strefling AM, Swanson K, Eng LF (1986) Reaction of LBs with antibodies to phosphorylated and nonphosphorylated neurofilaments. Neurosci Lett 64: 253–258
Galloway PG, Grundke-Iqbal I, Iqbal K, Perry G (1988) LBs contain epitopes both shared and distinct from Alzheimer neurofibrillary tangles. J Neuropathol Exp Neurol 47: 654–663
Gaspar P, Berger B, Febvret A, Vigny A, Krieger-Poulet M, Borri-Voltattorni C (1987) Tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex: a novel catecholaminergic group? Neurosci Lett 80: 257–262
Gentleman SM, Williams B, Royston MC, Jagoe R, Clinton J, Perry RH, Ince PG, Allsop D, Polak JM, Roberts GW (1992) Quantification of βA4 protein deposition in the medial temporal lobe: a comparison of Alzheimer's disease and senile dementia of the Lewy body type. Neurosci Lett 142: 9–12
Gibb WRG, Esiri MM, Lees AJ (1987) Clinical and pathological features of diffuse cortical Lewy body disease (Lewy body dementia). Brain 110: 1131–1153
Goldman JE, Yen S-H, Chiu F-C, Peress NS (1983) LBs of Parkinson's disease contain neurofilament antigens. Science 221: 1082–1084
Greenberg SG, Davies P (1990) A preparation of Alzheimer paired helical filaments that displays distinct τ proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA 87: 5827–5831
Haltia M, Ghiso J, Wisniewski T, Kiuru S, Miller D, Frangione B (1991) Gelsolin variant and amyloid co-occur in a case of Alzheimer's with LBs. Neurobiol Aging 12: 313–316
Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, Thal L, Pay MM, Hofstetter R, Klauber M, Rice V, Butters N, Alford M (1990) Lewy body variant of Alzheimer's disease: a clinical and pathological entity. Neurology 40: 1–8
Hill WD, Lee VM-Y, Hurtig HI, Murray JM, Trojanowski JQ (1991) Epitopes located in spatially separate domains of each neurofilament subunit are present in Parkinson's disease LBs. J Comp Neurol 309: 150–160
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnoses of idiopathic Parkinson's disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181–184
Ince P, Irving D, MacArthur F, Perry RH (1991) Quantitative neuropathological study of Alzheimer-type pathology in the hippocampus: comparison of senile dementia of Alzheimer type, senile dementia of Lewy body type, Parkinson's disease and nondemented elderly control patients. J Neurol Sci 106: 142–152
Kosaka K, Yoshimura M, Ikeda K, Budka H (1984) Diffuse type of Lewy body disease: progressive dementia with abundant cortical LBs and senile changes of varying degree. A new disease? Clin Neuropathol 3: 185–192
Kosaka K, Tsuchiya K, Yoshimura M (1988) Lewy body disease with and without dementia: a clinicopathologic study of 35 cases. Clin Neuropathol 7: 299–305
Kosik K, Joachim CL, Selkoe DJ (1986) Microtubule associated proteins tau (τ) is a major component of paired helical filaments in Alzheimer's disease. Proc Natl Acad Sci USA 83: 4044–4048
Kukulis RO, Martin-Vasallo P, Peress NS (1989) LBs in tyrosine hydroxylase synthesizing neurons of the human cerebral cortex. Neurosci Lett 106: 49–54
Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y (1988) LBs are ubiquitinated: a light and electron microscopic immunocytochemical study. Acta Neuropathol (Berl) 75: 345–353
Kwak S, Masaki T, Ishiuri S, Sugita H (1991) Multicatalytic proteinase is present in LBs and neurofibrillary tangles in diffuse Lewy body disease brains. Neurosci Lett 128: 21–24
Lee VM-Y, Balin BJ, Otvos L, Torjanowski JQ (1991) A68: a major subunit of paired helical filaments and derivatized froms of normal tau. Science 231: 675–678
Lewis DA, Campbel MJ, Foote SL, Morrison JH (1986) The monoaminergic innervation of primate neocortex. Hum Neurobiol 5: 181–188
Love S, Saitoh T, Quijada S, Cole GM, Terry RD (1988) Alz-50, ubiquitin and tau immunoreactivity of neurofibrillary tangles, Pick bodies and LBs. J Neuropathol Exp Neurol 47: 393–405
Lowe J, Blanchard A, Morrell K (1988) Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson's disease, Pick's disease and Alzheimer's disease, as well as Rosenthal fibers in cerebellar astrocytomas, cytoplasmic bodies in muscle and Mallory bodies in alcoholic liver disease. J Pathol 155: 9–15
Lowe J, McDermott H, Landon M, Mayer RJ, Wilkinson KD (1990) Ubiquitin carboxy-terminal hydrolase (PGP9.5) is selectively present in ubiquitinated inclusion bodies characteristic of human neurodegenerative disease. J Pathol 161: 153–160
Manetto V, Perry G, Tabaton M, Mulvihill P, Fried VA, Smith HT, Gambetti P, Autillo-Gambetti L (1988) Ubiquitin is associated with abnormal cytoplasmic filaments characteristic of neurodegenerative disorders. Proc Natl Acad Sci USA 85: 4501–4505
Mark ME, Sage JI, Dickson DW, Schwartz KO, Duvoisin RC (1992) Levodopa nonresponsive Lewy body parkinsonism: clinicopathologic study of two cases. Neurology 42: 1323–1327
Nakashima S, Ikuta F (1984) Tyrosine hydroxylase proteins in LBs of Parkinsonian and senile brains. J Neurosci Res 64: 91–96
Pappolla MA (1986) LBs of Parkinson's disease: immune electron microscopic demonstration of neurofilament antigens in constituent filaments. Arch Pathol Lab Med 110: 1160–1163
Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK (1990) Senile dementia of the Lewy body type: a clinically and neuropathologically distinct form of Lewy body dementia in the elderly. J Neurol Sci 95: 119–139
Pollanen MS, Bergeron C, Weyer L (1992) Detergentinsoluble Lewy body fibrils share epitopes with neurofilament and tau. J Neurochem 58: 1953–1956
Pollanen MS, Bergeron C, Weyer L (1993) Deposition of detergent-resistent neurofilaments into Lewy body fibrils. Brain Res 603: 121–124
Pollanen MS, Dickson DW, Bergeron C (1993) Pathology and biology of the Lewy body. J Neuropathol Exp Neurol 52: 183–191
Schmidt ML, Lee VM-Y, Trojanowski JQ (1991) Comparative epitope analysis of neuronal cytoskeletal proteins in Alzheimer's disease senile plaque neurites and neuropil threads. Lab Invest 64: 352–357
Schmidt ML, Murray JM, Lee VM-Y, Hill WD, Wertkin A, Trojanowski JQ (1991) Epitope map of neurofilament protein domains on cortical and peripheral nervous system LBs. Am J Pathol 139: 53–65
Torack RM, Morris JC (1990) Tyrosine hydroxylase-like (TH) immunoreactivity in human mesolimbic system. Neurosci Lett 116: 75–80
Torack RM, Morris JC (1992) Tyrosine hydroxylase-like (TH) immunoreactivity in Parkinson's disease and Alzheimer's disease. J Neural Transm [Park Dis Dement] 4: 165–171
Wisniewski T, Haltia M, Ghiso J, Frangione B (1991) LBs are immunoreactive with antibodies to gelsolin related amyloid-Finnish type. Am J Pathol 138: 1077–1083
Wolozin BL, Pruchnicki A, Dickson DW, Davies P (1986) A neuronal antigen in the brains of patients with Alzheimer's disease. Science 232: 648–650
Wu E, Lipton RG, Dickson DW (1992) Amyloid angiopathy in diffuse Lewy body disease. Neurology 42: 2131–2135
Yamada T, McGeer PL, McGeer EG (1992) LBs in Parkinson's disease are recognized by antibodies to complement proteins. Acta Neuropathol 84: 100–104
Yoshimura N, Yoshimura I, Asada M, Hayashi S, Fukushima Y, Kudo H (1988) Juvenile Parkinson's disease with widespread LBs in the brain. Acta Neuropathol 77: 213–218
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health, grant Nos. NIA AG60803, AG09215, AG10124
Rights and permissions
About this article
Cite this article
Dickson, D.W., Schmidt, M.L., Lee, V.M.Y. et al. Immunoreactivity profile of hippocampal CA2/3 neurites in diffuse Lewy body disease. Acta Neuropathol 87, 269–276 (1994). https://doi.org/10.1007/BF00296742
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00296742