Summary
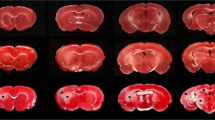
Carotid arteries were occluded bilaterally for 15 min in two groups of Mongolian gerbils. The first group received 100% oxygen during the first 3 h of reperfusion. During that period, room air was given to the second group. After 3 h, both groups received room air. Brains of gerbils that died within 14 days after occlusion were removed, fixed in formalin and embedded in paraffin. Gerbils that survived 15–28 days were perfused with formalin before their brains were removed and embedded in paraffin. Adjacent, serially cut sections were stained with luxol fast blue (LFB)-H&E, cresyl violet, according to the Bodian method, or immunocytochemically with antisera raised against myelin basic protein (MBP) and glial fibrillary acidic protein (GFAP). In brain sections of gerbils receiving 3 h of 100% oxygen, there were circumscribed white matter lesions in the corpus striatum, lateral thalamus, mesencephalon and posterior limb of the internal capsule. Myelin sheaths were swollen, fragmented and were less intensely stained by MBP antiserum. MBP and LFB-stained myelin fragments were present extracellularly and in macrophages. Many axons in these areas appeared undamaged. Previously described ischemic changes were found in gray matter and some areas of white matter in both groups. However, neurons in the deeper laminae of the cerebral cortex appeared to be better preserved in gerbils given oxygen. The results suggest that hyperoxia, if present immediately after transient brain ischemia, may damage myelin more severely than other cellular elements.
Similar content being viewed by others
References
Brannan TS, Weinberger J, Knott P, Taff I, Kaufmann H, Togasaki D, Nieves-Rosa J, Maker H (1987) Direct evidence of acute, massive striatal dopamine release in gerbils with unilateral strokes. Stroke 18:108–110
Chan OH, Yurko M, Fishman RA (1982) Phospholipid degradation and cellular edema induced by free radicals in brain cortical slices. J Neurochem 38:525–531
Djuricic BM, Paschen W, Bosma HJ, Hossman KA (1983) Biochemical changes during graded brain ischemia in gerbils. Part 1. Global biochemical alterations. J Neurol Sci 58:25–36
Du Bois M, Bowman PD, Goldstein GW (1985) Cell proliferation after ischemic infarction in gerbil brain. Brain Res 347:245–252
Du Bois M, Bowman PD, Goldstein GW (1985) Cell proliferation after ischemic injury in gerbil brain: an immunocytochemical and autoradiographic study. Cell Tissue Res 242:17–23
Garcia J, Lossinsky AS, Kauffman FC, Conger KA (1978) Neuronal ischemic injury: light microscopy, ultrastructure and biochemistry. Acta Neuropathol (Berl) 43:85–95
Heikkila R, Cohen G (1971) Inhibition of biogenic amine uptake by hydrogen peroxide: a mechanism for toxic effects of 6-hydroxydopamine. Science 172:1257–1258
Hsu SM, Raine L, Fanger H (1981) The use of avidin-biotinperoxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Ito U, Spatz M, Walker JT Jr, Klatzo I (1975) Experimental cerebral ischemia in Mongoliam gerbils. I. Light microscopic observations. Acta Neuropathol (Berl) 32:209–223
Ito U, Go KG, Walker JT Jr, Spatz M, Klatzo I (1976) Experimental cerebral ischemia in Mongolian gerbils. III. Behaviour of the blood-brain barrier. Acta Neuropathol (Berl) 34:1–6
Itoyama Y, Sternberger NH, Kies MW, Cohen SR, Richardson EP Jr, Webster H deF (1980) Immunocytochemical method to identify myelin basic protein in oligodendroglia and myelin sheaths of the human nervous system. Ann Neurol 7:157–166
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–70
Konat GW, Wiggins RC (1985) Effect of reactive oxygen species on myelin membrane proteins. J Neurochem 45: 1113–1118
Kontos HA, Wei EP, Ellis EF, Dietrich WD, Povlishock JT (1981) Prostaglandins in physiological and in certain pathological responses of the cerebral circulation. Fed Proc 40:2326–2330
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312:159–163
Mickel HS (1975) Multiple sclerosis: a new hypothesis. Perspect Biol Med 18:363–374
Mickel HS (1978) Peroxidized arachidonic acid effects on human platelets and its proposed role in the induction of damage to white matter. In: Kabara JJ (ed) The pharmacological effects of lipids. AOCS Monogr No 5, Champaign, pp 179–180
Mickel HS (1985) Biological effects of lipid peroxides: lipid peroxidation hypothesis of the etiology of multiple sclerosis. In: Kabara JJ (ed) The pharmacological effects of lipids. II. AOCS Monogr No 13, Champaign, pp 215–246
Mickel HS, Vaishnav YN, Kempski O, von Lubitz D, Weiss JF, Feuerstein G (1987) Breathing 100% oxygen after global brain ischemia results in increased lipid peroxidation and increased mortality. Stroke 18:426–430
Mickel HS, Kempski O, Parisi JE, Feuerstein G, Webster H deF (1988) Breathing 100% O2 after global brain ischemia results in more extensive white matter damage than breathing air. Stroke 19:140
Mickel HS, Starke-Reed PE, Oliver CN (1989) Rapid correction of severe hyponatremia results in myelinolysis, brain protein oxidation, and altered blood chemistries. Ann Emerg Med 18:460–461
O'Brien JS, Fillerup DL, Mead JL (1964) Quantification of fatty acid and fatty aldehyde composition of ethanolamine, choline and serine glycerophosphatides in human cerebral gray and white matter. J Lipid Res 5:320–338
Piccozi P, Todd NV, Crockard HA (1985) Regional bloodbrain barrier permeability changes after restoration of blood flow in post-ischemic gerbil brains. A quantitative study. J Cereb Blood Flow Metab 5:10–16
Rivett AJ, Roseman JE, Oliver CN, Levine RL, Stadtman ER (1985) Covalent modification of proteins by mixed-function oxidation: Recognition by intracellular proteases. In: Khairallah EA, Bond JS, Bird JW (eds) Intracellular protein catabolism. Alan R Liss, New York, pp 317–328
Rosenblum WI (1987) Hydroxyl radical mediates the endothelium-dependent relaxation produced by bradykinin in mouse cerebral arterioles. Circ Res 61:601–603
Sato S, Quarles RH, Brady RO (1982) Susceptibility of the myelin-associated glycoprotein and basic protein to a neutral protease in highly purified myelin from human and rat brain. J Neurochem 39:97–105
Tayarani I, Chaudiere J, LeFauconnier J-M, Bourre J-M (1987) Enzymatic protection against peroxidative damage in isolated brain capillaries. J Neurochem 48:1399–1402
Yanagihara T, Yoshimine T, Morimoto K, Yamamoto K, Homburger HA (1985) Immunohistochemical investigation of cerebral ischemia in gerbils. J Neuropathol Exp Neurol 44:203–215
Yusa T (1986) Hydrogen peroxide generation in rat brain in-vivo correlates with oxygen pressure. Jpn J Anesthesiol 35:1077–1082
Yusa T, Beckman JS, Crapo JD, Freeman BA (1987) Hyperoxia increases hydrogen peroxide production by brain in-vivo. J Appl Physiol 63:353–358
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mickel, H.S., Kempski, O., Feuerstein, G. et al. Prominent white matter lesions develop in Mongolian gerbils treated with 100% normobaric oxygen after global brain ischemia. Acta Neuropathol 79, 465–472 (1990). https://doi.org/10.1007/BF00296104
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00296104