Abstract
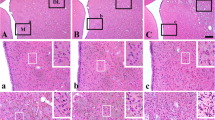
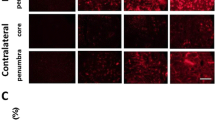
The degree of transient ischemic damage in the cerebral hemisphere is different according to duration of transient ischemia and cerebral regions. Mongolian gerbils show various lesions in the hemisphere after transient unilateral occlusion of the common carotid artery (UOCCA) because they have different types of patterns of anterior and posterior communicating arteries. We examined differential regional damage in the ipsilateral hemisphere of the gerbil after 30 min of UOCCA by using 2,3,5-triphenyltetrazolium chloride (TTC) staining, cresyl violet (CV) Nissl staining, Fluoro-Jade B (F-J B) fluorescence staining, and NeuN immunohistochemistry 5 days after UOCCA. In addition, regional differences in reactions of astrocytes and microglia were examined using GFAP and Iba-1 immunohistochemistry. After right UOCCA, neurological signs were assessed to define ischemic symptomatic animals. Moderate symptomatic gerbils showed several infarcts, while mild symptomatic gerbils showed selective neuronal death/loss in the primary motor and sensory cortex, striatum, thalamus, and hippocampus 5 days after UOCCA. In the areas, morphologically changed GFAP immunoreactive astrocytes and Iba-1 immunoreactive microglia were found, and their numbers were increased or decreased according to the damaged areas. In brief, our results demonstrate that 30 min of UOCCA in gerbils produced infarcts or selective neuronal death depending on ischemic severity in the ipsilateral cerebral cortex, striatum, thalamus and hippocampus, showing that astrocytes and microglia were differently reacted 5 days after UOCCA. Taken together, a gerbil model of 30 min of UOCCA can be used to study mechanisms of infarction and/or regional selective neuronal death/loss as well as neurological dysfunction following UOCCA.






Similar content being viewed by others
References
Ahn JH, Shin BN, Park JH et al (2016) Long-term observation of neuronal degeneration and microgliosis in the gerbil dentate gyrus after transient cerebral ischemia. J Neurol Sci 363:21–26
Bae EJ, Chen BH, Shin BN et al (2015) Comparison of immunoreactivities of calbindin-D28k, calretinin and parvalbumin in the striatum between young, adult and aged mice, rats and gerbils. Neurochem Res 40:864–872
Barakat R, Redzic Z (2016) The Role of Activated Microglia and Resident Macrophages in the Neurovascular Unit during Cerebral Ischemia: Is the Jury Still Out? Med Princ Pract 25(Suppl 1):3–14
Baron JC, Yamauchi H, Fujioka M, Endres M (2014) Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab 34:2–18
Canazza A, Minati L, Boffano C, Parati E, Binks S (2014) Experimental models of brain ischemia: a review of techniques, magnetic resonance imaging, and investigational cell-based therapies. Front Neurol 5:19
Candelario-Jalil E, Alvarez D, Merino N, Leon OS (2003) Delayed treatment with nimesulide reduces measures of oxidative stress following global ischemic brain injury in gerbils. Neurosci Res 47:245–253
Choudhury GR, Ding S (2016) Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiol Dis 85:234–244
Du XY, Zhu XD, Dong G et al (2011) Characteristics of circle of Willis variations in the mongolian gerbil and a newly established ischemia-prone gerbil group. ILAR J 52:E1–E7
Ejaz S, Williamson DJ, Ahmed T et al (2013) Characterizing infarction and selective neuronal loss following temporary focal cerebral ischemia in the rat: a multi-modality imaging study. Neurobiol Dis 51:120–132
Emmrich JV, Ejaz S, Neher JJ, Williamson DJ, Baron JC (2015) Regional distribution of selective neuronal loss and microglial activation across the MCA territory after transient focal ischemia: quantitative versus semiquantitative systematic immunohistochemical assessment. J Cereb Blood Flow Metab 35:20–27
Ha Park J, Yoo KY, Hye Kim I et al (2016) Hydroquinone Strongly Alleviates Focal Ischemic Brain Injury via Blockage of Blood-Brain Barrier Disruption in Rats. Toxicol Sci 154:430–441
Hanyu S, Ito U, Hakamata Y, Yoshida M (1993) Repeated unilateral carotid occlusion in Mongolian gerbils: quantitative analysis of cortical neuronal loss. Acta Neuropathol 86:16–20
Hanyu S, Ito U, Hakamata Y, Yoshida M (1995) Transition from ischemic neuronal necrosis to infarction in repeated ischemia. Brain Res 686:44–48
Hanyu S, Ito U, Hakamata Y, Nakano I (1997) Topographical analysis of cortical neuronal loss associated with disseminated selective neuronal necrosis and infarction after repeated ischemia. Brain Res 767:154–157
Ishibashi S, Kuroiwa T, Endo S, Okeda R, Mizusawa H (2003) Neurological dysfunctions versus regional infarction volume after focal ischemia in Mongolian gerbils. Stroke 34:1501–1506
Ishibashi S, Kuroiwa T, Katsumata N, Yuan S, Endo S, Mizusawa H (2004) Extrapyramidal motor symptoms versus striatal infarction volume after focal ischemia in mongolian gerbils. Neuroscience 127:269–275
Ito U, Hakamata Y, Watabe K, Oyanagi K (2014) Mannitol infusion immediately after reperfusion suppresses the development of focal cortical infarction after temporary cerebral ischemia in gerbils. Neuropathology 34:360–369
Katchanov J, Waeber C, Gertz K et al (2003) Selective neuronal vulnerability following mild focal brain ischemia in the mouse. Brain Pathol 13:452–464
Kim DW, Cho JH, Cho GS et al (2015) Hyperthermic preconditioning severely accelerates neuronal damage in the gerbil ischemic hippocampal dentate gyrus via decreasing SODs expressions. J Neurol Sci 358:266–275
Kim JY, Park J, Chang JY, Kim SH, Lee JE (2016) Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp Neurobiol 25:241–251
Kirino T, Sano K (1980) Changes in the contralateral dentate gyrus in Mongolian gerbils subjected to unilateral cerebral ischemia. Acta Neuropathol 50:121–129
Kirino T, Sano K (1984) Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol 62:201–208
Kitagawa K, Matsumoto M, Matsushita K et al (1996) Ischemic tolerance in moderately symptomatic gerbils after unilateral carotid occlusion. Brain Res 716:39–46
Lee JC, Ahn JH, Lee DH et al (2013) Neuronal damage and gliosis in the somatosensory cortex induced by various durations of transient cerebral ischemia in gerbils. Brain Res 1510:78–88
Lee JC, Park JH, Ahn JH et al (2016a) New GABAergic Neurogenesis in the Hippocampal CA1 Region of a Gerbil Model of Long-Term Survival after Transient Cerebral Ischemic Injury. Brain Pathol 26:581–592
Lee TK, Park JH, Ahn JH et al (2016b) Pretreated duloxetine protects hippocampal CA1 pyramidal neurons from ischemia-reperfusion injury through decreases of glial activation and oxidative stress. J Neurol Sci 370:229–236
Levy DE, Brierley JB, Plum F (1975) Ischaemic brain damage in the gerbil in the absence of'no-reflow'. J Neurol Neurosurg Psychiatry 38:1197–1205
Matsui T, Mori T, Tateishi N et al (2002) Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part I: enhanced astrocytic synthesis of S-100β in the periinfarct area precedes delayed infarct expansion. J Cereb Blood Flow Metab 22:711–722
Matsumoto M, Hatakeyama T, Akai F, Brengman JM, Yanagihara T (1988) Prediction of stroke before and after unilateral occlusion of the common carotid artery in gerbils. Stroke 19:490–497
Miyajima N, Ito M, Rokugawa T et al (2018) Detection of neuroinflammation before selective neuronal loss appearance after mild focal ischemia using [(18)F]DPA-714 imaging. EJNMMI Res 8:43
Momosaki S, Ito M, Yamato H et al (2017) Longitudinal imaging of the availability of dopamine transporter and D2 receptor in rat striatum following mild ischemia. J Cereb Blood Flow Metab 37:605–613
Murakami K, Kondo T, Kawase M, Chan PH (1998) The development of a new mouse model of global ischemia: focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res 780:304–310
Naritomi H (1991) Experimental basis of multi-infarct dementia: memory impairments in rodent models of ischemia. Alzheimer Dis Assoc Disord 5:103–111
Ohno K, Ito U, Inaba Y (1984) Regional cerebral blood flow and stroke index after left carotid artery ligation in the conscious gerbil. Brain Res 297:151–157
Oostveen JA, Timby K, Williams LR (1992) Prediction of cerebral ischemia by ophthalmoscopy after carotid occlusion in gerbils. Stroke 23:1588–1593
Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG (2007) Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci 27:4253–4260
Park JH, Cho JH, Ahn JH et al (2018) Neuronal loss and gliosis in the rat striatum subjected to 15 and 30 minutes of middle cerebral artery occlusion. Meta Brain Dis 33:775–784
Pulsinelli WA (1985) Selective neuronal vulnerability: morphological and molecular characteristics. Prog Brain Res 63:29–37
Radtke-Schuller S, Schuller G, Angenstein F, Grosser OS, Goldschmidt J, Budinger E (2016) Brain atlas of the Mongolian gerbil (Meriones unguiculatus) in CT/MRI-aided stereotaxic coordinates. Brain Struct Funct 221(Suppl 1):1–272
Rodriguez Cruz Y, Mengana Tamos Y, Munoz Cernuda A et al (2010) Treatment with nasal neuro-EPO improves the neurological, cognitive, and histological state in a gerbil model of focal ischemia. TheScientificWorldJournal 10:2288–2300
Schmued LC, Hopkins KJ (2000) Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 874:123–130
Schmued LC, Albertson C, Slikker W Jr (1997) Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res 751:37–46
Sicard KM, Fisher M (2009) Animal models of focal brain ischemia. Exp Transl Stroke Med 1:7
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (NRF-2015M3A9B6066835), by 2017 Research Grant from Kangwon National University (No. 520170438), by "Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01329401)" Rural Development Administration, Republic of Korea, and by Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01321101)" Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The experimental protocol of this study was approved by the Institutional Animal Care and Use Committee (IACUC) at Kangwon National University (approval no. KW-180124-1) and is in accordance with the guidelines following current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, The National Academies Press, 8th Ed., 2011).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ahn, J.H., Song, M., Kim, H. et al. Differential regional infarction, neuronal loss and gliosis in the gerbil cerebral hemisphere following 30 min of unilateral common carotid artery occlusion. Metab Brain Dis 34, 223–233 (2019). https://doi.org/10.1007/s11011-018-0345-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0345-9