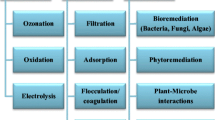
Abstract
Heavy metals and dyes are released from different industries which cause adverse effects on the environment. It is a persistent problem because metals are not biodegradable. Conventional treatment of heavy metals and dyes is not cost-effective and also produces large amounts of hazardous waste and mud. Plant-microbe synergism is an essential portion of our earthly bionetwork; recently many researchers have explored this field to understand the plant-microbe-heavy metal/dye interactions. These interactions have many applications in the field of phytoremediation technology. The technique rhizorestitution is a particular type of phytoremediation that can solve the problems of sites contaminated with heavy metals and dyes. Rhizospheric and endophytic microbiome connected with plant system have the potential of biodegrading the organic compounds in the contaminated site. Potential metabolites such as 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, indole-3-acetic acid (IAA), organic acids, some volatiles, etc. are synthesized by plant-associated microorganisms (e.g., mycorrhizae, bacteria); these metabolites are involved in many biogeochemical progressions which operate in rhizoplane and rhizospheric zone. Plant-associated microbes have acidifying reduction and chelating power. Plant-microbe interactions enhance the uptake of heavy metals using many biological and geochemical processes, which mainly includes uptake, translocation, immobilization, chelation, precipitation, solubilization, volatilization, and complex formation of heavy metals, and finally lead to phytorestitution. In general, the plant-microbe interaction increases the effectiveness of phytoremediation process by altering the heavy metal gathering or accumulation and dye in plant tissue parts. In this chapter, we are focusing on the different types of plant-microbe interactions for the bioremediation of synthetic dyes and different types of heavy metals. Focus will be on different plant-microbe interaction-based bioremediation methods to eliminate the dyes and heavy metals in polluted sites.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abhilash PC, Powell JR, Singh HB, Singh BK (2012) Plant-microbe interactions: novel applications for exploitation in multipurpose remediation technologies. Trends Biotechnol 30:416–420
Abou-Shanab RA, Delorme TA, Angle JS, Chaney RL, Ghanem K, Moawad H (2003) Phenotypic characterization of microbes in the rhizosphere of Alyssum murale. Int J Phytoremediation 5:367–379
Abou-Shanab RAI, Angle JS, Chaney RL (2006) Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biol Biochem 38:2882–2889
Arshad M, Saleem M, Hussain S (2007) Perspectives of bacterial ACC deaminase in phytoremediation. Trends Biotechnol 25:356–362
Aslantas R, Cakmakci R, Sahin F (2007) Effect of plant growth promoting rhizobacteria on young apple tree growth and fruit yield under orchard conditions. Sci Hortic 11:371–377
Barak RI, Nur IS, Okon YA, Henis YI (1982) Aerotactic response of Azospirillum brasilense. J Bacteriol 152:643–649
Barak R, Nur I, Okon Y (1983) Detection of chemotaxis in Azospirillum brasilense. J Appl Bacteriol 54:399–403
Baskaralingam P, Pulikesi M, Elango D, Ramamurthi V, Sivanesan S (2006) Adsorption of acid dye onto organobentonite. J Hazard Mater 128:138–144
Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI, Davies WJ (2009) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol 181:413–423
Benson DR, Silvester WB (1993) Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol Rev 57:293–319
Boddey RM, Baldani VL, Baldani JI, Döbereiner J (1986) Effect of inoculation of Azospirillum spp. on nitrogen accumulation by field-grown wheat. Plant Soil 95:109–121
Bonkowski M, Villenave C, Griffiths B (2009) Rhizosphere fauna: the functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 321:213–233
Brazil GM, Kenefick L, Callanan M, Haro A, de Lorenzo V, Dowling DN (1995) Construction of a rhizosphere pseudomonas with potential to degrade polychlorinated biphenyls and detection of bph gene expression in the rhizosphere. Appl Environ Microbiol 61:1946–1952
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154:275–304
Buée M, De Boer W, Martin F, Van Overbeek L, Jurkevitch E (2009) The rhizosphere zoo: an overview of plant associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil 321:189–212
Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EV, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838
Burd GI, Dixon DG, Glick BR (2000) Plant growth promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46:237–245
Butani N, Jobanputra J, Bhatiya P, Patel R (2013) Recent biological technologies for textile effluent treatment. Int Res J Biol Sci 2:77–82
Cakmakçi R, Dönmez F, Aydın A, Şahin F (2006) Growth promotion of plants by plant growth-promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biol Biochem 38:1482–1487
Cao RX, Ma LQ, Chen M, Singh SP, Harris WG (2003) Phosphate induced metal immobilization in a contaminated site. Environ Pollut 122:19–28
Chakraborty U, Chakraborty B, Basnet M (2006) Plant growth promotion and induction of resistance in Camellia sinensis by Bacillus megaterium. J Basic Microbiol 46:186–195
Chaudhry Q, Zandstra MB, Gupta S, Joner EJ (2005) Utilizing the synergy between plants and rhizosphere organisms to enhance breakdown of organic pollutants in the environment. Environ Sci Pollut Res 12:34–48
Chisholm JE, Jones GC, Purvis OW (1987) Hydrated copper oxalate, moolooite, in lichens. Mineral Mag 51:715–718
Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124:803–814
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Cripps C, Bumpus JA, Aust SD (1990) Biodegradation of azo and heterocyclic dyes by Phanerochaete chrysosporium. Appl Environ Microbiol 56:1114–1118
Daneshvar N, Ayazloo M, Khataee AR, Pourhassan M (2007) Biological decolorization of dye solution containing malachite green by microalgae Cosmarium sp. Bioresour Technol 98:1176–1182
Das A, Prasad R, Bhatnagar K, Lavekar GS, Varma A (2006) Synergism between medicinal plants and microbes. In: Chauhan AK, Varma A (eds) Microbes: health and environment, vol 3. IK International-India, New Delhi, pp 13–64
Dell’Amico E, Cavalca L, Andreoni V (2008) Improvement of Brassica napus growth under cadmium stress by cadmium resistant rhizobacteria. Soil Biol Biochem 40:74–84
Dimkpa CO, Svatoš A, Dabrowska P, Schmidt A, Boland W, Kothe E (2008a) Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 74:19–25
Dimkpa CO, Svatoš A, Merten D, Büchel G, Kothe E (2008b) Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can J Microbiol 54:163–172
Dimkpa CO, Merten D, Svatoš A, Büchel G, Kothe E (2009a) Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol Biochem 41:154–162
Dimkpa CO, Merten D, Svatos A, Büchel G, Kothe E (2009b) Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol 107:1687–1696
Dobbelaere S, Vanderleyden J, Okon Y (2003) Plant growth promoting effects of diazotrophs in the rhizosphere. Crit Rev Plant Sci 22:107–149
Dodds PN, Rathjen JP (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nat Rev Genet 11:539–548
Doran PM (2009) Application of plant tissue cultures in phytoremediation research: incentives and limitations. Biotechnol Bioeng 103:60–76
El-Rahim WM (2006) Assessment of textile dye remediation using biotic and abiotic agents. J Basic Microbiol 46:318–328
Erkel C, Kube M, Reinhardt R, Liesack W (2006) Genome of Rice Cluster I archaea—the key methane producers in the rice rhizosphere. Science 313:370–372
Ernst WHO (1996) Bioavailability of heavy metals and decontamination of soil by plants. Appl Geochem 11:163–167
Forchetti G, Masciarelli O, Alemano S, Alvarez D, Abdala G (2007) Endophytic bacteria in sunflower (Helianthus annuus L.): isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl Microbiol Biotechnol 76:1145–1152
Fox RT (ed) (2000) Armillaria root rot: biology and control of honey fungus. Intercept, Andover
Gans J, Wolinsky M, Dunbar J (2005) Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 309:1387–1390
Garcıa JA, Domenech J, Santamarıa C, Camacho M, Daza A, Mañero FJ (2004) Growth of forest plants (pine and holm-oak) inoculated with rhizobacteria: relationship with microbial community structure and biological activity of its rhizosphere. Environ Exp Bot 52:239–251
Gerhardt KE, Huang XD, Glick BR, Greenberg BM (2009) Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci 176:20–30
Gianfreda L, Rao MA (2004) Potential of extra cellular enzymes in remediation of polluted soils: a review. Enzym Microb Technol 35:339–354
Gianinazzi S, Gollotte A, Binet MN, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Glick BR (2003) Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21:383–393
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:367–374
Goltapeh EM, Danesh YR, Prasad R, Varma A (2008) Mycorrhizal Fungi: what we know and what should we know? In: Varma A (ed) Mycorrhiza, 3rd edn. Springer-Verlag, Berlin Heidelberg, pp 3–27
Guan LL, Kanoh K, Kamino K (2001) Effect of exogenous siderophores on iron uptake activity of marine bacteria under iron limited conditions. Appl Environ Microbiol 67:1710–1717
Guarino C, Sciarrillo R (2017) Effectiveness of in situ application of an Integrated Phytoremediation System (IPS) by adding a selected blend of rhizosphere microbes to heavily multi-contaminated soils. Ecol Eng 99:70–82
Guo JH, Qi HY, Guo YH, Ge HL, Gong LY, Zhang LX, Sun PH (2004) Biocontrol of tomato wilt by plant growth-promoting rhizobacteria. Biol Control 29:66–72
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–1
Hall PG, Krieg NR (1984) Application of the indirect immuno peroxidase stain technique to the flagella of Azospirillum brasilense. Appl Environ Microbiol 47:433
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Hartmann A, Zimmer W (1994) Physiology of Azospirillum. Azospirillum/plant associations. CRC Press, Boca Raton, pp 15–39
Heinrich D, Hess D (1985) Chemotactic attraction of Azospirillum lipoferum by wheat roots and characterization of some attractants. Can J Microbiol 31:26–31
Herridge DF (2013) Rhizobial inoculants. GRDC
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152
Hock B (ed) (2012) Fungal associations IX, 2nd edn. Esser K (ed). Springer, Berlin/Heidelberg/New York/Dordrecht/London. https://doi.org/10.1007/978-3-642-30826-0. ISBN: 978-3-642-30825-3
Hu MR, Chao YP, Zhang GQ, Xue ZQ, Qian S (2009) Laccase-mediator system in the decolorization of different types of recalcitrant dyes. J Ind Microbiol Biotechnol 36:45–51
Huang XD, El-Alawi Y, Penrose DM, Glick BR, Greenberg BM (2004) A multiprocess phytoremediation system for removal of polycyclic aromatic hydrocarbons from contaminated soils. Environ Pollut 130:465–476
Jetiyanon K, Kloepper JW (2002) Mixtures of plant growth-promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol Control 24:285–291
Jiang CY, Sheng XF, Qian M, Wang QY (2008) Isolation and characterization of a heavy metal resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal polluted soil. Chemosphere 72:157–164
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kabata-Pendias A (1992) Trace metals in soils in Poland-occurrence and behaviour. Soil Sci 140:53–70
Kabra AN, Khandare RV, Kurade MB, Govindar SP (2011) Phytoremediation of a sulphonated azo dye green HE4B by Glandularia pulchella (Sweet) Tronc. (Moss Verbena). Environ Sci Pollut Res 18:1360–1373
Kabra AN, Khandare RV, Govindwar SP (2013) Development of a bioreactor for remediation of textile effluent and dye mixture: a plant bacterial synergistic strategy. Water Res 47:1035–1048
Kapulnik Y (ed) (1991) Plant growth promoting rhizobacteria. Plant roots the hidden half. Marcel Dekker, New York, pp 347–362
Karaca S, Gürses A, Açıkyıldız M, Ejder M (2008) Adsorption of cationic dye from aqueous solutions by activated carbon. Microporous Mesoporous Mater 115:376–382
Khammas KM, Ageron E, Grimont PA, Kaiser P (1989) Azospirillum irakense sp. nov., a nitrogen-fixing bacterium associated with rice roots and rhizosphere soil. Res Microbiol 140:679–693
Khan AG (2005) Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J Trace Elem Med Biol 18:355–364
Khan MS, Zaidi A, Wani PA (2007) Role of phosphate solubilizing microorganisms in sustainable agriculture—a review. Agron Sustain Dev 27:29–43
Khandare R, Kabra A, Tamboli D, Govindwar S (2011) The role of Aster amellus. Lin. In the degradation of a sulfonated azo dye Remazol Red: a phytoremediation strategy. Chemosphere 82:1147–1154
Khandare RV, Rane NR, Waghmode TR, Govindwar SP (2012) Bacterial assisted phytoremediation for enhanced degradation of highly sulfonated diazo reactive dye. Environ Sci Pollut Res 19:1709–1718
Kiers ET, Denison RF (2008) Sanctions, cooperation, and the stability of plant rhizosphere mutualisms. Annu Rev Ecol Evol Syst 39:215–236
Kloepper JW, Schroth MN (1978) Plant growth promoting rhizobacteria on radishes. In: Proceedings of the 4th international conference on plant pathogenic bacteria, vol 2. pp 879–882
Kukier U, Peters CA, Chaney RL, Angle JS, Roseberg RJ (2004) The effect of pH on metal accumulation in two Alyssum species. J Environ Qual 32:2090–2102
Kulkarni AN, Kadam AA, Kachole MS, Govindwar SP (2014) Lichen Permelia perlata: a novel system for biodegradation and detoxification of disperse dye Solvent Red 24. J Hazard Mater 276:461–468
Kumar KV, Srivastava S, Singh N, Behl HM (2009) Role of metal resistant plant growth promoting bacteria in ameliorating fly ash to the growth of Brassica juncea. J Hazard Mater 170:51–57
Kurade MB, Waghmode TR, Govindwar SP (2011) Preferential biodegradation of structurally dissimilar dyes from a mixture by Brevibacillus laterosporus. J Hazard Mater 192:1746–1755
Lamm RB, Neyra CA (1981) Characterization and cyst production of azospirilla isolated from selected grasses growing in New Jersey and New York. Can J Microbiol 27:1320–1325
Ledin M, Krantz-Rulcker C, Allard B (1996) Zn, Cd and Hg accumulation by microorganisms, organic and inorganic soil components in multi compartment system. Soil Biol Biochem 28:791–799
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Lisci M, Monte M, Pacini E (2003) Lichens and higher plants on stone: a review. Int Biodeterior Biodegradation 51:1–7
Long XX, Chen XM, Wong JW, Wei ZB, Wu QT (2013) Feasibility of enhanced phytoextraction of Zn contaminated soil with Zn mobilizing and plant growth promoting endophytic bacteria. Trans Nonferrous Met Soc China 23:2389–2396
Lopez-de-Victoria G, Lovell CR (1993) Chemotaxis of Azospirillum species to aromatic compounds. Appl Environ Microbiol 59:2951–2955
Lopez-de-Victoria G, Fielder DR, Zimmer-Faust RK, Lovell CR (1994) Motility behavior of Azospirillum species in response to aromatic compounds. Can J Microbiol 40:705–711
Lucas Garcia JA, Probanza A, Ramos B, Barriuso J, Gutierrez Manero FJ (2004) Effects of inoculation with plant growth promoting rhizobacteria (PGPRs) and Sinorhizobium fredii on biological nitrogen fixation, nodulation and growth of Glycine max cv. Osumi. Plant Soil 267:143–153
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth promoting rhizobacteria. Anton Leeuw 86:1–25
Ma Y, Rajkumar M, Freitas H (2009) Isolation and characterization of Ni mobilizing PGPB from serpentine soils and their potential in promoting plant growth and Ni accumulation by Brassica spp. Chemosphere 75:719–725
Ma Y, Prasad MN, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69:220–228
Magalhaes FM, Baldani JI, Souto SM, Kuykendall JR, Dobereiner J (1983) New acid-tolerant Azospirillum species. An Acad Bras Cienc 55:417–430
McCormick MK, Whigham DF, O’Neill J (2004) Mycorrhizal diversity in photosynthetic terrestrial orchids. New Phytol 163:425–438
McCormick MK, Lee Taylor D, Juhaszova K, Burnett RK, Whigham DF, O’Neill JP (2012) Limitations on orchid recruitment: not a simple picture. Mol Ecol 21:1511–1523
Mishra AK, Cournoyer B, Dawson J, Jeannin P, Evtushenko L, Normand P, Orso S, Chapelon C (2010) Molecular phylogeny of the genus Frankia and related genera and emendation of the family Frankiaceae
Moens S, Michiels K, Keijers V, Van Leuven FR, Vanderleyden J (1995) Cloning, sequencing, and phenotypic analysis of laf1, encoding the flagellin of the lateral flagella of Azospirillum brasilense Sp7. J Bacteriol 177:5419–5426
Narasimhan K, Basheer C, Bajic VB, Swarup S (2003) Enhancement of plant-microbe interactions using a rhizosphere metabolomics-driven approach and its application in the removal of polychlorinated biphenyls. Plant Physiol 132:146–153
Nash TH (2008) Lichen biology, 2nd edn. Cambridge University Press, Cambridge, pp 5–6. isbn:978-0-521-69216-8
Nihorimbere V, Ongena M, Smargiassi M, Thonart P (2011) Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotechnol Agron Soc Environ 15(2):327
Okon Y (1994) Azospirillum/plant associations. CRC Press, Boca Raton, p 175
Oldroyd GE (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11:252–263
Olson PE, Reardon KF, Pilon-Smits EA (2003) Ecology of rhizosphere bioremediation. In: Phytoremediation: transformation and control of contaminants, pp 317–353
Pawlik-Skowronska B, Purvis OW, Pirszel J, Skowronski T (2006) Cellular mechanisms of Cu-tolerance in the epilithic lichen Lecanora polytropa growing at a copper mine. Lichenologist 38:267–275
Perrig D, Boiero ML, Masciarelli OA, Penna C, Ruiz OA, Cassán FD, Luna MV (2007) Plant growth promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Appl Microbiol Biotechnol 75:1143–1150
Pommer EH (1959) Über die Isolierung des Endophyten aus den Wurzelknöllchen Alnus glutinosa Gaertn. und über erfolgreiche Re-Infektionsversuche. Ber Deut Bot Ges 72:138–150
Prasad MNV, Freitas H, Fraenzle S, Wuenschmann S, Markert B (2010) Knowledge explosion in phytotechnologies for environmental solutions. Environ Pollut 158:18–23
Prasad R, Kumar M, Varma A (2015) Role of PGPR in soil fertility and plant health. In: Egamberdieva D, Shrivastava S, Varma A (eds) Plant Growth-Promoting Rhizobacteria (PGPR) and medicinal plants. Springer International Publishing, Switzerland, pp 247–260
Purvis OW (1984) The occurrence of copper oxalate in lichens growing on copper sulphide-bearing rocks in Scandinavia. Lichenologist 16:197–204
Raj SN, Deepak SA, Basavaraju P, Shetty HS, Reddy MS, Kloepper JW (2003) Comparative performance of formulations of plant growth promoting rhizobacteria in growth promotion and suppression of downy mildew in pearl millet. Crop Protect 22:579–588
Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ (2006) Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 62:741–748
Rasmussen HN (1995) Terrestrial orchids: from seed to mycotrophic plant. Cambridge University Press, Cambridge
Rasmussen HN, Whigham DF (1998) The underground phase: a special challenge in studies of terrestrial orchid populations. Bot J Linean Soc 126:49–64
Reinhold BA, Hurek TH, Fendrik IS (1985) Strain-specific chemotaxis of Azospirillum spp. J Bacteriol 162:190–195
Reinhold B, Hurek T, Fendrik I, Pot B, Gillis M, Kersters K, Thielemans S, De Ley J (1987) Azospirillum halopraeferens sp. nov., a nitrogen-fixing organism associated with roots of Kallar grass (Leptochloa fusca (L.) Kunth). Int J Syst Bacteriol 37:43–51
Ryu CM, Hu CH, Locy RD, Kloepper JW (2005) Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil 268:285–292
Sadasivan LA, Neyra CA (1985) Flocculation in Azospirillum brasilense and Azospirillum lipoferum: exopolysaccharides and cyst formation. J Bacteriol 163:716–723
Sadasivan LA, Neyra CA (1987) Cyst production and brown pigment formation in aging cultures of Azospirillum brasilense ATCC 29145. J Bacteriol 169:1670–1677
Saleem M, Moe LA (2014) Multitrophic microbial interactions for eco-and agro-biotechnological processes: theory and practice. Trends Biotechnol 32:529–537
Saleh S, Huang XD, Greenberg BM, Glick BR (2004) Phytoremediation of persistent organic contaminants in the environment. In: Applied bioremediation and phytoremediation. Springer, Berlin, pp 115–134
Salem HM, Eweida EA, Farag A (2000) Heavy metals in drinking water and their environmental impact on human health. In: ICEHM 2000. Cairo University, Giza, pp 542–556
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2009) Decolorization and biodegradation of textile dye Navy blue HER by Trichosporon beigelii NCIM-3326. J Hazard Mater 166:1421–1428
Saravanakumar D, Vijayakumar C, Kumar N, Samiyappan R (2007) PGPR-induced defense responses in the tea plant against blister blight disease. Crop Prot 26:556–565
Saroj S, Kumar K, Pareek N, Prasad R, Singh RP (2014) Biodegradation of azo dyes acid red 183, direct blue 15 and direct red 75 by the isolate Penicillium oxalicum SAR-3. Chemosphere 107:240–248
Sarret G, Manceau A, Cuny D, Van Haluwyn C, Déruelle S, Hazemann JL, Soldo Y, Eybert-Bérard L, Menthonnex JJ (1998) Mechanisms of lichen resistance to metallic pollution. Environ Sci Technol 32:3325–3330
Sawada H, Kuykendall LD, Young JM (2003) Changing concepts in the systematics of bacterial nitrogen-fixing legume symbionts. J Gen Appl Microbiol 49:155–179
Schröder P, Harvey PJ, Schwitzguébel J (2002) Prospects for the phytoremediation of organic pollutants in Europe. Environ Sci Pollut Res 9:1–3
Schuessler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: evolution and phylogeny. Mycol Res 105:1413–1421
Sekhar KC, Chary NS, Kamala CT, Vairamani M, Anjaneyulu Y, Balaram V, Sorlie JE (2006) Environmental risk assessment studies of heavy metal contamination in the industrial area of Kattedan, India—a case study. Hum Ecol Risk Assess 12:408–422
Sharma J, Ogram AV, Al-Agely A (2015) Mycorrhizae: implications for environmental remediation and resource conservation
Shrivastava S, Prasad R, Varma A (2014) Anatomy of root from eyes of a microbiologist. In: Morte A, Varma A (eds) Root Engineering, vol 40. Springer-Verlag, Berlin Heidelberg, pp 3–22
Siddiqui ZA, Mahmood I (2001) Effects of rhizobacteria and root symbionts on the reproduction of Meloidogyne javanica and growth of chickpea. Bioresour Technol 79:41–45
Sikora RA, Schafer K, Dababat AA (2007) Modes of action associated with microbially induced in planta suppression of plant–parasitic nematodes. Australas Plant Pathol 36:124–134
Slampova A, Smela D, Vondrackova A, Jancarova I, Kuban V (2001) Determination of synthetic colorants in foodstuffs. Chem List 95:163–168
Smith DL (2005) Intracellular and extracellular PGPR: commonalities and Gray distinctions in the plant-bacterium signaling processes. Soil Biol Biochem 37:395–412
Smith SE, Read DJ (2010) Mycorrhizal symbiosis. Academic, London
Soares GM, Teresa M, Amorim P, Lageiro M, Costa-Ferreira M (2006) Pilot-scale enzymatic decolorization of industrial dyeing process wastewater. Text Res J 76:4–11
Sturz AV, Christie BR, Nowak J (2000) Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci 19:1–30
Sylvia D, Fuhrmann J, Hartel P, Zuberer D (2005) Principles and applications of soil microbiology. Pearson Education, New Jersey
Sziderics AH, Rasche F, Trognitz F, Sessitsch A, Wilhelm E (2007) Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can J Microbiol 53:1195–1202
Tal S, Okon Y (1985) Production of the reserve material poly-β-hydroxybutyrate and its function in Azospirillum brasilense Cd. Can J Microbiol 31:608–613
Tal S, Smirnoff P, Okon Y (1990) The regulation of poly-β-hydroxybutyrate metabolism in Azospirillum brasilense during balanced growth and starvation. Microbiology 136:1191–1196
Tamboli DP, Kagalkar AN, Jadhav MU, Jadhav JP, Govindwar SP (2010) Production of polyhydroxyhexadecanoic acid by using waste biomass of Sphingobacterium sp. ATM generated after degradation of textile dye Direct Red 5B. Biol Resour Technol 101:2421–2427
Tarrand JJ, Krieg NR, Döbereiner J (1978) A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol 24:967–980
Taylor DL, Bruns TD, Leake JR, Read DJ (2002) Mycorrhizal specificity and function in myco-heterotrophic plants. In: Mycorrhizal ecology. Springer, Berlin, pp 375–413
Tedersoo L, May TW, Smith ME (2010) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263
Timmis KN, Pieper DH (1999) Bacteria designed for bioremediation. Trends Biotechnol 17:201–204
Togo CA, Mutambanengwe CC, Whiteley CG (2008) Decolourisation and degradation of textile dyes using a sulphate reducing bacteria (SRB)–biodigester microflora co-culture. Afr J Biotechnol 7:114–121
Tokala RK, Strap JL, Jung CM, Crawford DL, Salove MH, Deobald LA, Bailey JF, Morra MJ (2002) Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl Environ Microbiol 68:2161–2171
Tor A, Cengeloglu Y (2006) Removal of congo red from aqueous solution by adsorption onto acid activated red mud. J Hazard Mater 138:409–415
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Villacieros M, Whelan C, Mackova M, Molgaard J, Sanchez-Contreras M, Lloret J (2005) Polychlorinated biphenyl rhizoremediation by Pseudomonas fluorescence F113 derivatives, using a Sinorhizobium meliloti nod system to drive bph gene expression. Appl Environ Microbiol 71:2687–2694
Welbaum GE, Sturz AV, Dong Z, Nowak J (2004) Managing soil microorganisms to improve productivity of agro-ecosystems. Crit Rev Plant Sci 23:175–193
Weyens N, van der Lelie D, Taghavi S, Newman L, Vangronsveld J (2009a) Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol 27:591–598
Weyens N, van der Lelie D, Taghavi S, Vangronsveld J (2009b) Phytoremediation: plant–endophyte partnerships take the challenge. Curr Opin Biotechnol 20:248–254
Whiting SN, de Souza MP, Terry N (2001) Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens. Environ Sci Technol 35:3144–3150
Wrobel D, Boguta A, Ion RM (2001) Mixtures of synthetic organic dyes in a photoelectronic cell. J Photochem Photobiol A 138:7–22
Wu SC, Cheung KC, Luo YM, Wong MH (2006) Effects of inoculation of plant growth-promoting rhizobacteria on metal uptake by Brassica juncea. Environ Pollut 140:124–135
Wuchter C, Abbas B, Coolen MJ, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl GJ, Middelburg JJ, Schouten S (2006) Archaeal nitrification in the ocean. Proc Natl Acad Sci 103:12317–12322
Yee DC, Maynard JA, Wood TK (1998) Rhizoremediation of trichloroethylene by a recombinant, root-colonizing Pseudomonas fluorescens strain expressing toluene ortho-monooxygenase constitutively. Appl Environ Microbiol 64:112–118
Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63:968–989
Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64:991–997
Zakry FA, Shamsuddin ZH, Rahim KA, Zakaria ZZ, Rahim AA (2012) Inoculation of Bacillus sphaericus UPMB-10 to young oil palm and measurement of its uptake of fixed nitrogen using the 15N isotope dilution technique. Microbes Environ 27:257–262
Zhang Y, Burris RH, Ludden PW, Roberts GP (1997) Regulation of nitrogen fixation in Azospirillum brasilense. FEMS Microbiol Lett 152(2):195–204
Zhulin IB, Armitage JP (1993) Motility, chemokinesis, and methylation-independent chemotaxis in Azospirillum brasilense. J Bacteriol 175:952–958
Acknowledgment
Gurpreet Kaur is thankful to the Department of Science and Technology for Award of Inspire Faculty (IFA-12-CH-41) and PURSE Grant II. Rajeev Kumar is thankful to DST, SERB/F/8171/2015-16, as well as UGC (F. No. 194-2/2016 IC) for providing financial support. Varsha Dogra is thankful to UGC for Junior Research Fellowship.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dogra, V., Kaur, G., Kumar, R., Prakash, C. (2018). The Importance of Plant-Microbe Interaction for the Bioremediation of Dyes and Heavy Metals. In: Kumar, V., Kumar, M., Prasad, R. (eds) Phytobiont and Ecosystem Restitution. Springer, Singapore. https://doi.org/10.1007/978-981-13-1187-1_22
Download citation
DOI: https://doi.org/10.1007/978-981-13-1187-1_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1186-4
Online ISBN: 978-981-13-1187-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)