Abstract
Plants encounter various nanomaterials (NMs) as pesticides and fertilizers. It is also possible that nanomaterials reach plants as waste from consumer products and industry. The effects of such NMs on plants have been widely studied, and both positive and negative effects of NMs on plant growth and development have been reported. Recent metabolomics studies suggest that nanoparticles affect the concentration of secondary metabolites in plants by modulating reactive nitrogen/oxygen species, gene expression, and signaling pathways. Secondary metabolites are plant compounds that accumulate in plants through their secondary metabolism. To date, more than 200,000 defined structures of secondary metabolites have been identified, among which many of them possess antibacterial, antifungal, antiviral, anti-inflammatory, hepatoprotective, antidepressant, antioxidant, neuroprotective, and anticancer properties. The application of elicitors is a simple strategy to increase the production of secondary metabolites in plant cell and tissues. The ability of nanomaterials to induce plant secondary metabolism has recently been exploited in the elicitation of pharmaceutically important compounds from various plant species. The ability of different NMs to induce the accumulation of different classes of compounds in the same plant species has also been accomplished. The molecular mechanisms behind the effects of NMs on plant secondary metabolism revealed the putative genes involved in NM-mediated elicitation of various plant compounds in several reports. This chapter reviews the current understanding of the effects of nanoparticles on plant secondary metabolism and the elicitation of pharmacologically important compounds from plant species.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In this era of nanotechnology, nanomaterials (NMs) are finding applications in various fields, including science, industry, medicine, and agriculture. Several consumer products, medicines, fertilizers, pesticides, cosmetics, food packings, paints and electronics containing NMs are already on the market.
Plants are exposed to NMs through various pathways. NMs can move to plants as NM-containing wastes that are released into the environment by industries and consumer products in water and soil. The predicted concentration of some nanoparticles (NPs) in soil is: silver (Ag): 0.91–1.8 ng/kg; titanium oxide (TiO2): 0.09–0.24 μg/kg; zinc oxide (ZnO): 0.01–0.03 μg/kg (Sun et al. 2014). On the other hand, recent advances in agriculture use formulations containing NMs such as fertilizers, fungicides and pesticides. The concentration-dependent response to NPs varies greatly among different plant species, which has been reported, for example, for NPs from ZnO: 40–1200 ppm, (Mosquera-Sánchez et al. 2020; Sadak and Bakry 2020) cerium oxide (CeO2): 125–500 ppm, (Rico et al. 2014) copper oxide (CuO): 200–400 ppm, (Wang et al. 2019) and gold (Au): 5 ppm (Kang et al. 2016) to show the effect of nanofertilizers and pesticides. Effective concentrations for plant protection applications range from 2 to 2000 ppm for Ag NPs alone across plant species and pathogens (Elmer and White 2018). Thus, while the application of nanotechnology is expected to revolutionize agriculture, NMs that enter the environment directly as agrochemicals or indirectly as industrial or household wastes are proving to be pollutants with unknown consequences for plants.
Previous studies on various model plant species and crops have shown that NMs affect plant growth and development both positively and negatively depending on their concentration. However, it is known that the biologically relevant concentration of NMs strongly depends on their metallic core, physicochemical properties, substrate and plant species. NMs are known to interfere with metabolic processes and lead to the formation of reactive oxygen species (ROS)/reactive nitrogen species (RNS), damage the structure and function of cell membranes, and reduce enzyme activities and DNA synthesis. Recent literature also suggests that plant secondary metabolism is also affected by NMs.
Secondary metabolism is crucial for plants as they play an indispensable role in plant survival: as protection against herbivores and pathogenic microbes, as signals for symbiotic interactions of plants with beneficial microorganisms, as allelopathic agents in natural habitats for protection against competitors, as physical and chemical barriers against abiotic stressors such as UV radiation, and as endogenous regulators of plant growth regulators.
The small molecular products that are biosynthesized in plants through their secondary metabolic pathways are called plant secondary metabolites. These compounds are generally classified as terpenes, steroids, phenols, flavonoids and alkaloids and are derived from primary metabolites or as an intermediate in the primary metabolic pathway (Chandran et al. 2020; Pang et al. 2021). Plant secondary metabolites play an important role in plant defense mechanisms against biotic and abiotic stresses (Khare et al. 2020; Mahajan et al. 2020). In particular, phenylpropanoids are involved in the regulation of oxidative stress, free ion chelation, cell wall lignification, and plant defense (Agati et al. 2012). In addition, secondary metabolites are also known to be involved in pest defense (Barlow et al. 2017; Stevenson 2020), signal transduction in plant–microbe symbiosis (Adedeji and Babalola 2020) and plant innate immunity (Piasecka et al. 2015).
Apart from their beneficial effects in plants, many secondary metabolites are economically important as medicines, flavors and fragrances, dyes and pigments, pesticides and food additives. Useful remedies from herbal medicine are due to the presence of various secondary metabolites (Chandran et al. 2020). For example, a recent study showed that 12 pure compounds from Clerodendranthus spicatus (Thunb.) C. Y. Wu ex H. W. Li, an herb widely used in traditional Chinese medicine for the treatment of kidney inflammation, gout, and dysuria, promoted the excretion of uric acid (Chen et al. 2020). More than 500 secondary metabolites have been reported from 46 species of the genus Lycopodium, and these secondary metabolites have been shown to have several medically important bioactivities, including neuroprotective, anti-inflammatory, anti-cancer, antiviral, and antimicrobial activities (Wang et al. 2021).
The quantity of secondary metabolites produced by natural biosynthesis in plants is limited to meet the growing demand of the pharmaceutical industry. Thus, development of alternative biotechnological approaches is necessary to boost production of secondary metabolites (Thakur et al. 2019). Elicitation is one of the most commonly used techniques to enhance the biosynthesis of secondary metabolites (Thakur et al. 2019; Yazdanian et al. 2021).
In recent years, NMs have emerged as novel triggers for inducing biosynthesis of bioactive compounds in plants (Shakya et al. 2019; Rivero-Montejo et al. 2021). Ag NP treatment increased artemisinin content by 3.9-fold in 20-day-old hairy root cultures of Artemisia annua L. (Zhang et al. 2013). Hydroponically grown Bacopa monnieri L. treated with copper-based NPs (Cu) improved antioxidant capacity and showed hormetic increase in the content of saponins, alkaloids, flavonoids and phenols (Lala 2020). Celastrol, a therapeutically important phytochemical, was increased in adventitious and hairy root cultures of Celastrus paniculatus Willd. after treatment with Ag NP (Moola et al. 2021). The elicitation of various classes of bioactive secondary metabolites in Hypericum perforatum L. cell suspension cultures treated with various metal (Ag, Au, Cu, Pd) and metal oxide (CeO2, CuO, TiO2, ZnO) NPs has been recently reported (Kruszka et al. 2022).
In this chapter, we discuss the effects of NMs on secondary metabolism in plants, focusing on signaling events and key medicinal agents that are enhanced by NPs.
2 Plant’s Response to Nanomaterials
Exposure to NMs has been found to induce changes in various physiological, morphological and developmental processes of plants. In general, plant metabolism can be divided into primary (associated with energy and biosynthesis of building blocks) and secondary (more specialized molecules) metabolism (Erb and Kliebenstein 2020). Primary metabolites consist of the products of photosynthesis, glycolysis, the tricarboxylic acid cycle (TCA cycle), biosynthesis of amino acids, lipids, and some natural polymers. Cu NPs minimized the negative effects of drought stress on photosynthetic pigments and promoted plant growth, development and grain yield in Zea mays L. (Van Nguyen et al. 2021). Foliar application of silica (SiO2) and ZnO NPs in Cucumis sativus L. significantly increased chlorophyll content and various amino acids and modulated carbon metabolic processes in leaves (Li et al. 2021a). In contrast to primary metabolism, secondary metabolism yields structurally diverse and specialized metabolites, such as phenylpropanoids (polyphenols, flavonoids, anthocyanins, xanthones, stilbenes), terpenes, polyketides, prenylated phloroglucinols, alkaloids, and organosulfur compounds (glucosinolates, thioesters). These metabolites play a role as phytoalexins, phytoanticides and phytoncides (defense systems against many biotic stresses), antioxidants (control ROS), chelators (scavenging free metal ions), UV protectants, growth regulators, and factors against abiotic stresses (Feng et al. 2021b; Nobahar et al. 2021). Various NPs including iron (Fe), cerium (Ce), and SiO2, altered secondary metabolite content in lettuce and pepper seedlings (Kalisz et al. 2021).
2.1 Impact of NPs on Precursors of Secondary Metabolism
The effects of different types of NMs on precursors of secondary metabolites have been analyzed in detail in algal, monocotyledonous, and dicotyledonous plant models (Table 6.1). Many studies have captured the effects of NMs on the pentose phosphate pathway, glycolysis, and the TCA cycle, and have linked carbohydrates and organic acids to these processes. The upregulation of these metabolic pathways and compounds is related to defense mechanisms and their additional roles as chelators and osmoprotectors (Li et al. 2019; Nobahar et al. 2021). Moreover, Ag (Chavez Soria et al. 2017), CuO (Zhao et al. 2017a), Cu(OH)2 (Zhao et al. 2018a), CdO (Večeřová et al. 2016), CeO2 (Salehi et al. 2018), graphene-based (Hu and Zhou 2015; Ouyang et al. 2015; Chen et al. 2021), WS2 (Yuan et al. 2018) and fullerols (Zhao et al. 2019) affected the fatty acids and lipid compositions of various plant species such as Arabidopsis thaliana (L.) Heynh., C. sativus, Z. mays, Hordeum vulgare L. and Phaseolus vulgaris L.
Amino acid metabolism is an important bridge between primary and secondary metabolites. Many amino acids are important precursors in the biosynthesis of alkaloids (e.g., arginine, lysine, ornithine, phenylalanine, proline, tryptophan, tyrosine), glucosinolates (e.g. methionine, leucine, isoleucine, phenylalanine, tryptophan), and phenylpropanoids (e.g., phenylalanine and tyrosine) (Barros and Dixon 2020; Jan et al. 2021). Ag, CuO, Cu(OH)2 NPs and Ag+, Cu2+ ions stimulated accumulation of aromatic amino acids in C. sativus tissues, Z. mays (Zhao et al. 2016a, 2018b, 2018a; Zhang et al. 2018), A. thaliana (Chavez Soria et al. 2019) and Triticum aestivum L. (Feng et al. 2021a). The biosynthesis of other amino acids was up regulated by, ZnO (Li et al. 2021b), C60 fullerols (Zhao et al. 2019) and graphene NPs (Chen et al. 2021; Hu and Zhou 2015).
2.2 Impact of NPs on Secondary Metabolism
A number of studies reported the effects of NPs on plant secondary metabolism (Table 6.2). Accumulation of shikimate and phenylpropanoid pathway products was observed in cucumber and maize after foliar application of Cu(OH)2 (Zhao et al. 2018a) in wheat exposed to Ag (Feng et al. 2021a), in pepper exposed to SiO2 or Fe2O3 (Kalisz et al. 2021) and in A.thaliana exposed to CuO (Chavez Soria et al. 2019) NPs. On the other hand, the amount of phenylpropanoids in lettuce, spinach, cucumber, and barley were decreased by Cu(OH)2 (Zhao et al. 2016b, 2017b), CeO2 (Zhang et al. 2019b), soil application of CuO (Huang et al. 2019), and CdO (Večeřová et al. 2016). Relatively low doses of CeO2 NPs induced metabolic reprogramming by affecting flavonoids and phenolic compounds in roots and leaves of P. vulgaris (Salehi et al. 2020).
The concentration of benzoic acid and gallic acid was increased, while the content of hydroxycinnamic acid derivatives was reduced in C. sativus when exposed to CuO NPs (Huang et al. 2019). In Solanum lycopersicum L., more anthocyanins and fewer flavonoids were formed after treatment with MWCT (McGehee et al. 2017).
Metal and metal oxide NPs have got impact on the biosynthesis of phenylpropanoids in the Hypericum perforatum L. cells (Kruszka et al. 2022). Metal nanoparticles (Ag, Au, Cu and Pd) increased accumulation of xanthones, prenylated xanthones and beznophenones and reduced levels of flavonoids and hydroxycinnamic acid derivatives in cells. In contrast to this, the level of flavonoids was increased in biomass by the CuO nanoparticles treatment.
NMs have altered the metabolism of alkaloids, a group of compounds that possesses high biological value as defense metabolites (Erb and Kliebenstein 2020). Salehi et al. (2018) reported that the concentration of (s)-corytuberine, laudanosine, and precursors of naphthylisoquinoline alkaloids decreased, while the content of demecolcine, β-caconine, and tropionone increased in P. vulgaris after foliar application of CeO2 NPs. Accumulation of taxane and tropane alkaloids was reported after Ag NPs treatment (Shakeran et al. 2015; Jamshidi and Ghanati 2017) and hyoscyamine and scopolamine after exposure to ZnO NPs (Asl et al. 2019). Metabolome and transcriptome analyses have shown that the biosynthesis of isoquinoline alkaloids was downregulated by MWCT in S. lycopersicum (McGehee et al. 2017) and GOQD (graphene oxide quantum dots) in Chlorella vulgaris Beijerinck (Kang et al. 2019).
Camalexin is a major indole phytoalexin produced by A. thaliana in response to biotic and abiotic stresses. Application of Ag NPs induced the accumulation of this compound (Kruszka et al. 2020). Transcriptomic analysis showed that metabolism of tryptophan (camalexin precursor) is upregulated by exposure of A. thaliana to PVP-Ag NPs (Zhang et al. 2019a). The same research shows that exposure upregulates the biosynthesis of glucosinolates—precursors of isothiocyanates (biologically active form).
3 Molecular Mechanisms of Nanomaterials-Induced Secondary Metabolic Changes
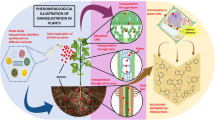
Secondary metabolite profiles in plants are dynamic and can change under biotic (pathogen and insect attack) and abiotic (UV radiation, drought, temperature, salinity and heavy metals.) stress conditions. In particular, the interaction between NMs and plants leads to overproduction of ROS, oxidative stress, membrane structure impairment, alteration of antioxidant activities, altered secondary metabolism, hormone pathways, and signal transduction (Fu et al. 2014; Hossain et al. 2015). For example, secondary metabolic changes were associated with increased levels of ROS, phenylalanine ammonia lyase (PAL) and polyphenol oxidase (PPO) in A. thaliana exposed to 250 and 1000 mg/L CeO2 and indium oxide (In2O3) NPs, respectively (Ma et al. 2016). Moreover, they also alter the expression of genes related to cell division, cell organization, electron transport, and biotic and abiotic stress pathways (Landa et al. 2012; Van Aken 2015). The molecular mechanisms associated with changes of secondary metabolites triggered by NMs are summarized in Fig. 6.1.
Schematic diagram showing various cellular responses in response to NMs. NMs cause oxidative stress through overproduction of ROS, activation of the antioxidant defense system, lipid peroxidation, membrane damage, calcium bursts, activation of MAPK signaling pathways, and altered secondary metabolism in plants. Upward pointing arrows indicate increased abundance and downward pointing arrows indicate decreased abundance in the plant cell. Abbreviations: SOD, superoxide dismutase; APX, ascorbate peroxidase; GST, glutathione transferase; GR, glutathione reductase; DHAR, dehydroascorbate reductase; PAL, phenylalanine ammonia lyase; PPO, polyphenol oxidase. (Figure constructed by G. Franklin and P. Shakya)
3.1 Reactive Oxygen Species
ROS is the most rapid response of plants to all stresses and plays a dual role in both triggering the defense system and enhancing cell damage or disruption of signal transduction (Dat et al. 2000). NMs are known to induce ROS in plants (Marslin et al. 2017; Ranjan et al. 2021). The induction of ROS has been observed in both apoplast and chloroplast, preceded by intracellular calcium and MAPK signaling mechanisms (Marslin et al. 2017). Moreover, the molecular aspects of NPs-induced ROS on cell wall-related processes and secondary metabolism have been studied in detail for all stimulatory and inhibitory effects in plants (Berni et al. 2019). It was found that the effect of NPs on the plant system is concentration dependent. Higher concentrations were found to be toxic while lower concentrations resulted in beneficial effects (Jalil and Ansari 2019).
The ROS mechanism triggered by NPs to induce oxidative stress has been studied in different plant systems. In A. thaliana, the accumulation of ROS was induced by exogenous application of 100–5000 mg/L Ag NPs. These Ag NPs activate Ca2+ and ROS signals by inducing a transient increase in Ca2+ and direct oxidation of the major plant antioxidant, L-ascorbic acid (Sosan et al. 2016). In Allium cepa L. treatment with 0–80 mg/L Ag NPs led to the formation of ROS, resulting in DNA structural damage and eventual cell death (Panda et al. 2011). Treatment with Ag NPs altered proteins involved in redox regulation and sulfur metabolism in Eruca sativa Mill. roots (Vannini et al. 2013). Moreover, the formation of ROS was observed in Spirodela polyrhiza L. by inhibiting ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity and photoprotective capacity of PSII in the presence of Ag NPs (Jiang et al. 2017). Another study demonstrated the role of NiO NPs in H. vulgare by reporting that the overproduction of ROS led to oxidative stress and increased lipid peroxidation. However, simultaneous treatment of SiO2 NP with NiO resulted in an antioxidant response with decreased lipid peroxidation, highlighting the protective role of nano-SiO2 (Soares et al. 2018). Another study shows that the phytotoxic potential of cobalt oxide (Co3O4) NPs reduces seed germination, root growth, DNA and mitochondrial damage, oxidative stress and cell death in eggplant, while it increases ROS, membrane potential and nitric oxide (NO) (Faisal et al. 2016). Besides generating ROS, Ag and Ag+ NPs coated with polyvinylpyrrolidone (PVP) promote gene expression of stress-related genes in A. thaliana (Kaveh et al. 2013).
3.2 Calcium Ion Signaling
During various stresses, Ca2+ ions act as second messengers and provide Ca2+ ion channels for plant adaptation to adverse conditions (Tuteja and Mahajan 2007). The interaction of fullerene C60 nanocrystals (nano-C60) suspended in water with Ca2+/calmodulin-dependent protein kinase II (CaMKII) has been shown to modulate Ca2+ signal transduction function (Miao et al. 2014). In addition, Ag NPs bind to calcium receptors, Ca2+ ion channels, and calcium-sodium ATP pumps, thereby regulating cell metabolism. Ag NPs in Oryza sativa L. have been found to be involved in Ca2+ ion regulation and signaling, protein degradation, cell wall synthesis, transcription, oxidative stress tolerance, cell division, and apoptosis (Mirzajani et al. 2014). Proteomic studies also revealed the abundance of superoxide dismutase (SOD), L-ascorbate peroxidase (APX) and glutathione transferase (GST) in detoxification or oxidative reaction pathway (Mirzajani et al. 2014). Reports showed the role of NO in increasing cytosolic Ca2+ ions using Nicotiana plumbaginifolia L. cells and it also stimulates the activity of protein kinases during physiological processes (Lamotte et al. 2006).
3.3 Phytohormone Signaling
Plant metabolism is highly influenced by hormone regulation during plant growth, which mediates numerous responses to plant stresses (Santner et al. 2009). Several reports have shown the significant influence of NPs on plant hormones. For example, Fe2O3 NP uptake had a significant effect on IAA and ABA content in roots of transgenic and non-transgenic rice (Gui et al. 2015). Similarly, CeO2 NPs have a differential effect on indole-3-acetic acid (IAA), abscisic acid (ABA) and gibberellic acid (GA) in leaves and roots of transgenic and conventional Bt cotton compared to the control group (Nhan et al. 2015). Thin-walled carbon nanotubes (CNTs) treatment reduced the growth of O. sativa seedlings by decreasing the content of endogenous plant hormones such as IAA, GA, IPA, JA, BR and ABA (Hao et al. 2016). In A. thaliana, the response to ZnO NPs is associated with a decrease in growth, cytokinins and auxins in apices. Moreover, a higher dose led to an increase in the levels of ABA and SA, while it suppressed the levels of JA (Vankova et al. 2017). Similarly, Ag NPs were found to inhibit ethylene perception (ET) by hindering ET biosynthesis in A. thaliana (Syu et al. 2014).
3.4 Nitric Oxide (NO) Signaling
NO is a universal signaling molecule that plays an important role in nanomaterial-triggered changes in plant secondary metabolism. For example, NO burst leads to the accumulation of saponins and artemisinin during fungal attack (Zhang et al. 2012). In Pisum sativum L., NO showed protection against Ag NP induced phytotoxicity through increased superoxide dismutase (SOD) and ascorbate peroxidase (APX) activity and reduced glutathione reductase (GR) and dehydroascorbate reductase (DHAR) activities (Tripathi et al. 2017). On the other hand, CdO NPs showed a significant effect on primary metabolism of barley plants with an increase in total amino acids in roots and leaves and a decrease in saccharides in roots, but had no effect on secondary metabolites (Večeřová et al. 2016).
4 Applications of Nanomaterial-Induced Secondary Metabolic Changes
4.1 NPs as Biostimulants
Plant biostimulation is a process that leads to changes in plant metabolism in order to use available environmental resources more efficiently, increase tolerance to environmental stresses, and increase yield (Juárez-Maldonado et al. 2019). NPs are used as novel biostimulants to promote plant growth under stress conditions. Stimulation of secondary metabolites such as alkaloids, terpenoids, phenolic compounds, glucosinolates and flavonoids reduces the deleterious effects of environmental stress in plants (Rajput et al. 2021). For example, increased melatonin synthesis by application of ZnO NPs helped in controlling drought-induced damage in Z. mays (Sun et al. 2020). Melatonin is a secondary metabolite and is known to improve stress tolerance in plants by stimulating antioxidant activities (Marioni et al. 2008; Debnath et al. 2020). The quality, visual attractiveness and nutritional properties of Punica granatum L. sap have been found to be affected by the reduction of bioactive compounds such as anthocyanins and punicalagin under drought stress (Mena et al. 2013). Spraying leaves with selenium NPs increased phenolic content and improved the quality of drought-affected fruits of P. granatum (Zahedi et al. 2021).
4.2 NPs as Elicitors of Phytopharmaceuticals
Controlled elicitation is a strategy to increase the production of important secondary metabolites. As described in the previous sections, plants recognize different types of NMs and induce their secondary metabolism, which opens a new opportunity to improve the production of pharmaceutically important compounds in medicinal plants (Marslin et al. 2017; Shakya et al. 2019; Kruszka et al. 2020; Rivero-Montejo et al. 2021). Elicitation of several classes of secondary metabolites such as glucosinolates, terpenes and alkaloids have been reported to be obtained using NPs. The chemical structure of some pharmaceutically important secondary metabolites elicited using NMs is shown in Fig. 6.2.
Some of the pharmaceutically important secondary metabolites elicited from medicinal plants using NMs; (1) naringenin, (2) apigenin (R1 = H, R2 = OH, R3 = H, R4 = OH, R5 = H, R6 = H), (3) cirsimaritin (R1 = H, R2 = OMe, R3 = OMe, R4 = OH, R5 = H, R6 = H), (4) xanthomicrol (R1 = OMe, R2 = OMe, R3 = OMe, R4 = OH, R5 = H, R6 = H), (5) kaempferol (R1 = H, R2 = OH, R3 = H, R4 = OH, R5 = OH, R6 = H), (6) isokaempferide (R1 = H, R2 = OH, R3 = H, R4 = OH, R5 = OMe, R6 = H), (7) quercetin (R1 = H, R2 = OH, R3 = H, R4 = OH, R5 = OH, R6 = OH), (8) catechin, (9) chlorogenic acid, (10) cichoric acid, (11) atropin, (12) hyoscyamine, (13) scopolamine, (14) artemisinin, (15) carnosic acid, (16) tanshinone, (17) γ-mangostin, (18) garcinone B, (19) emodin, (20) fusaroskyrin. (Figure constructed by D. Kruszka)
4.2.1 Flavonoids
Flavonoids are natural bioactive compounds found predominantly in various parts of plants and have been attributed to various pharmacological and therapeutic properties (Panche et al. 2016). In Momordica charantia L., an increase in flavonoid concentration induced by 5 mg/L Ag NPs was observed (Chung et al. 2018c). Stimulation of Thymus daenensis Celak. plant cells with SWNT increased the total flavonoid content (Samadi et al. 2021). Quercetin is an important and abundant flavonoid from plants with rich pharmaceutical properties such as antitumor, anti-infective, anti-inflammatory and antioxidant activities (Qi et al. 2020). Increased quercetin content was observed in shoots and roots of Nigella arvensis L. treated with 50 mg/L NiO NPs (Modarresi et al. 2020). The level of several flavonoid aglycones like apigenin, kaempferol and quercetin was increased upon treatment with the Ag, Au, Cu and Pd NPs treatment, whereas flavonoid glucosides like quercetin 3-O-hexoside or quercetin 3-O-malonylhexoside was elicited by CuO NPs treatment in H. perforatum L. cell suspension cultures, (Kruszka et al. 2022). Anthocyanins are another subgroup of flavonoids and play an important role in the nutraceutical, pharmaceutical and food industries. After the application of ZnO NPs in the shooting culture of Lilium ledebourii (Baker) Boiss., an increase in anthocyanin concentration was observed, and the effect of polyphenol induction was dose-dependent (Chamani et al. 2015). Similarly, stimulation with SiO2 NPs increased the concentration of the anticancer flavonoids xanthomicrol, isocaempferide, and cirsimaritin in the hairy roots of Dracocephalum kotschyi Boiss. (Nourozi et al. 2019b). Treatment of D. kotschyi cell suspension cultures with Fe3O4 magnetite NPs increased the content of rosmarinic acid, naringin, carvacrol, rutin, quercetin, apigenin and thymol (Taghizadeh et al. 2021).
4.2.2 Phenolic Acids
Phenolic acids are an important group of plant secondary metabolites with a wide range of bioactivities, including anticancer, anti-inflammatory, neuroprotective, antioxidant, and antimicrobial activities (Kiokias et al. 2020). The phenolic acids, such as chlorogenic acid, coumaric acid, gallic acid, and tannic acid, were accumulated after the callus of Prunella vulgaris L. was exposed to Ag, Au, and Ag/Au NPs (Fazal et al. 2016). Moreover, Ag NPs induced the biosynthesis of phenolic acids more strongly than AgNO3 in the hairy root culture of Cucumis anguria L. (Chung et al. 2018b). Ag and Cu NPs stimulated the secretion of hydroxycinnamic acid and hydroxybenzoic acid derivatives from H. perforatum cells into media of cell suspension cultures (Kruszka et al. 2022).
4.2.3 Glucosinolates
Glucosinolates are a group of Sulphur-containing hydrophilic secondary metabolites found primarily in members of the Brassicaceae and related families (Poveda et al. 2020; Wu et al. 2021). Glucosinolates exhibit some pharmacological bioactivities such as anti-inflammatory, antimicrobial, cholinesterase inhibitory, antioxidant and anticancer properties (Maina et al. 2020). Ag NPs induced biosynthesis of glucosinolates, a group of compounds responsible for response to pathogen attack, in addition to phenolic compounds in seedlings of Brassica rapa L. (Thiruvengadam et al. 2015). Treatment of hairy roots of Chinese cabbage with CuO NPs increased the accumulation of glucosinolates (Chung et al. 2018c). Moreover, the extracts of hairy roots released showed higher antimicrobial activity compared to the control.
4.2.4 Terpenoids
Terpenes and terpenoids are biogenic volatile organic compounds of plant secondary metabolites with high biological activity against various human diseases (Kim et al. 2020). The production of monoterpenes (linalool and linalyl acetate) in shoot cultures of Mentha longifolia L. grown under the influence of Co (0.8 mg/L) and Cu (0.5 mg/L) NPs (Talankova-Sereda et al. 2016). They reported that the higher production of essential oils corresponded with the growth index (Talankova-Sereda et al. 2016). Artemisinin, one of the important pharmaceutical compounds used as antimalarials, was induced by 2.5 and 5 mg/L Co NPs in A. annua cell culture (Ghasemi et al. 2015). Similar results were obtained after stimulation of A. annua hairy root culture by Ag-SiO2 core–shell nanostructures (Zhang et al. 2013). A stimulatory effect of 8–21 nm Ag NPs on the increased production of diosgenin was observed in Trigonella foenum-graecum L. seedlings (Jasim et al. 2017). ZnO NPs (0.1–10 mg/L) increased the biosynthesis of rebaudioside-A and stevioside in shoot cultures of Stevia rebaudiana (Bert.), in addition to the induction of oxidative stress (Javed et al. 2017). Similarly, chitosan nanofibers and cellulose nanofibers increased the production of betulinic acid and betulin in cell suspension cultures of Betula pendula Roth (Vahide et al. 2021).
4.2.5 Alkaloids
Alkaloids are a large group of plant secondary metabolites with nitrogen atom(s) in their structure that exhibit a wide range of medicinally important bioactivities (Eguchi et al. 2019). Ag NPs induced the biosynthesis of atropine alkaloid in hair root culture of Datura metel L. and the highest level of atropine was detected after 48 h of treatment (Shakeran et al. 2015). This NP -based elicitor was better than AgNO3 and two other biotic elicitors (Staphylococcus aureus F. J. Rosenbach and Bacillus cereus Frankland & Frankland). Hyoscyamine and scopolamine levels were significantly increased 24 h after application of 450–1800 mg/L Fe2O3 NPs in the hairy root culture of Hyoscyamus reticulatus L. (Moharrami et al. 2017). SiO2 NPs triggered the production of tropane alkaloids (hyoscyamine and scopolamine) in hair root cultures of two Hyoscyamus species namely, H. reticulatus and H. pusillus L. (Hedayati et al. 2020). Cell suspension cultures of Corylus avellane produced more taxol and baccatin III after treatment with 5 mg/L Ag NPs (Jamshidi and Ghanati 2017). Available examples of the elicitation of pharmaceutically important secondary metabolites using NPs are summarized in Table 6.3.
4.2.6 Xanthones
Xanthones are bioactive secondary metabolites that possess antibacterial, antifungal activities, and could inhibit acetylcholinesterase, butyrylcholinesterase and tyrosinase (Badiali et al. 2018; Tusevski et al. 2018). Xanthones also possess neuroprotective activities (Xu et al. 2016; Velingkar et al. 2017). Ag, Au, Cu, Pd and CuO NPs stimulated accumulation of prenylated derivatives of xanthones (γ-mangostin, garcinone B and hyperxanthone C), whereas glycosylated xanthones (eg.: mangiferin, homomangiferin, neomangiferin) content was increased after Au, Cu and Pd NPs treatment in cell suspension system of H. perforatum L. (Kruszka et al. 2022).
4.2.7 Anthraquinones
Antidepressant activities of H. perforatum L. extracts are attributed to naphthodianthrones/ anthraquinones such as hypericin or pseudohypericin (Velingkar et al. 2017). Hypericin content was increased by TiO2-perlite nanocomposite treatment in H. perforatum L. shoot cultures (Ebadollahi et al. 2019). Emodin and emodin anthrone contents were respectively increased by Pd and CeO2 NPs treatment in H. perforatum L. cell suspension cultures (Kruszka et al. 2022). In the above study, a 98.6-fold increase of fusaroskyrin after Ag NP treatment was also reported.
5 Conclusion and Prospects
Plant secondary metabolites play an important role in plant’s fitness and adaptation. Therefore, alteration of secondary metabolism by NPs could affect crop quality and agricultural productivity. The pharmacological properties of several medicinal plants are attributed to the crude extracts or decoctions and not to the individual compounds. Therefore, any alteration in the secondary metabolism of medicinal plants would affect their pharmacological potential and market value. Among the numerous compounds accumulated in plants, many of them possess antibacterial, antifungal, antiviral, anti-inflammatory, hepatoprotective, antidepressant, antioxidant, neuroprotective and anticancer properties. A better understanding of the effects of NPs on plant secondary metabolism would allow us to develop strategies to help plants cope with the increasing presence of NPs in the environment and to develop new molecular pharmaceutical tools (Fig. 6.3).
The potential effects of secondary metabolic changes caused by NMs on other associated plant parameters. NMs can enter plants in both intentional and unintentional ways. Although changes in secondary metabolism could affect plants' ability to protect themselves against pathogens, herbivores, and adverse environmental conditions, as well as their ability to communicate with beneficial microbes, more research is needed to understand the exact consequences (Figure constructed by G. Franklin)
References
Abbasi BH, Zahir A, Ahmad W, Nadeem M, Giglioli-Guivarc’h N, Hano C, (2019) Biogenic zinc oxide nanoparticles-enhanced biosynthesis of lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Artif Cells, Nanomedicine, Biotechnol 47(1):1367–1373. https://doi.org/10.1080/21691401.2019.1596942
Adedeji AA, Babalola OO (2020) Secondary metabolites as plant defensive strategy: a large role for small molecules in the near root region. Planta 252(4):61. https://doi.org/10.1007/s00425-020-03468-1
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci 196:67–76. https://doi.org/10.1016/j.plantsci.2012.07.014
Ahmad B, Shabbir A, Jaleel H, Khan MMA, Sadiq Y (2018) Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr Plant Biol 13:6–15. https://doi.org/10.1016/j.cpb.2018.04.002
Ahmad MA, Javed R, Adeel M, Rizwan M, Ao Q, Yang Y (2020) Engineered ZnO and CuO Nanoparticles Ameliorate Morphological and Biochemical Response in Tissue Culture Regenerants of Candyleaf (Stevia rebaudiana). Molecules 25(6):1356. https://doi.org/10.3390/molecules25061356
Asl KR, Hosseini B, Sharafi A, Palazon J (2019) Influence of nano-zinc oxide on tropane alkaloid production, h6h gene transcription and antioxidant enzyme activity in Hyoscyamus reticulatus L. hairy roots. Eng Life Sci 19(1):73–89. https://doi.org/10.1002/elsc.201800087
Azeez L, Lateef A, Adebisi SA (2017) Silver nanoparticles (AgNPs) biosynthesized using pod extract of Cola nitida enhances antioxidant activity and phytochemical composition of Amaranthus caudatus Linn. Appl Nanosci 7(1):59–66. https://doi.org/10.1007/s13204-017-0546-2
Badiali C, De Angelis G, Simonetti G, Brasili E, de Tobaruela E, C, Purgatto E, Yin H, Valletta A, Pasqua G, (2018) Chitosan oligosaccharides affect xanthone and VOC biosynthesis in Hypericum perforatum root cultures and enhance the antifungal activity of root extracts. Plant Cell Rep 37(11):1471–1484. https://doi.org/10.1007/s00299-018-2317-2
Barlow SE, Wright GA, Ma C, Barberis M, Farrell IW, Marr EC, Brankin A, Pavlik BM, Stevenson PC (2017) Distasteful Nectar Deters Floral Robbery. Curr Biol 27(16):2552–2558.e3. https://doi.org/10.1016/j.cub.2017.07.012
Barros J, Dixon RA (2020) Plant Phenylalanine/Tyrosine Ammonia-lyases. Trends Plant Sci 25(1):66–79. https://doi.org/10.1016/j.tplants.2019.09.011
Berni R, Luyckx M, Xu X, Legay S, Sergeant K, Hausman JF, Lutts S, Cai G (2018) Guerriero G (2019) Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ Exp Bot 161:98–106. https://doi.org/10.1016/j.envexpbot.2018.10.017
Bhardwaj P, Goswami N, Narula P, Jain CK, Mathur A (2018) Zinc oxide nanoparticles (ZnO NP) mediated regulation of bacosides biosynthesis and transcriptional correlation of HMG-CoA reductase gene in suspension culture of Bacopa monnieri. Plant Physiol Biochem 130:148–156. https://doi.org/10.1016/j.plaphy.2018.07.001
Chamani E, Karimi Ghalehtaki S, Mohebodini M, Ghanbari A (2015) The effect of Zinc oxide nano particles and Humic acid on morphological characters and secondary metabolite production in Lilium ledebourii Bioss. Iran J Genet Plant Breed 4(2):11–19
Chandran H, Meena M, Barupal T, Sharma K (2020) Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol Reports (amsterdam, Netherlands) 26:e00450–e00450. https://doi.org/10.1016/j.btre.2020.e00450
Chavez Soria NG, Bisson MA, Atilla-Gokcumen GE, Aga DS (2019) High-resolution mass spectrometry-based metabolomics reveal the disruption of jasmonic pathway in Arabidopsis thaliana upon copper oxide nanoparticle exposure. Sci Total Environ 693:133443. https://doi.org/10.1016/j.scitotenv.2019.07.249
Chavez Soria NG, Montes A, Bisson MA, Atilla-Gokcumen GE, Aga DS (2017) Mass spectrometry-based metabolomics to assess uptake of silver nanoparticles by: Arabidopsis thaliana. Environ Sci Nano 4(10):1944–1953. https://doi.org/10.1039/c7en00555e
Chen W-D, Zhao Y-L, Sun W-J, He Y-J, Liu Y-P, Jin Q, Yang X-W, Luo X-D (2020) “Kidney Tea” and Its Bioactive Secondary Metabolites for Treatment of Gout. J Agric Food Chem 68(34):9131–9138. https://doi.org/10.1021/acs.jafc.0c03848
Chen Z, Niu J, Guo Z, Sui X, Xu N, Kareem HA, Hassan MU, Zhang Q, Cui J, Wang Q (2021) Integrating transcriptome and physiological analyses to elucidate the essential biological mechanisms of graphene phytotoxicity of alfalfa (Medicago sativa L.). Ecotoxicol Environ Saf 220:112348. https://doi.org/10.1016/j.ecoenv.2021.112348
Chung I-M, Rajakumar G, Subramanian U, Venkidasamy B, Thiruvengadam M (2019) Impact of Copper Oxide Nanoparticles on Enhancement of Bioactive Compounds Using Cell Suspension Cultures of Gymnema sylvestre (Retz.) R. Br. Appl Sci 9(10):2165. https://doi.org/10.3390/app9102165
Chung I-M, Rekha K, Rajakumar G, Thiruvengadam M (2018a) Production of bioactive compounds and gene expression alterations in hairy root cultures of chinese cabbage elicited by copper oxide nanoparticles. Plant Cell, Tissue Organ Cult 134(1):95–106. https://doi.org/10.1007/s11240-018-1402-0
Chung IM, Rajakumar G, Thiruvengadam M (2018b) Effect of silver nanoparticles on phenolic compounds production and biological activities in hairy root cultures of Cucumis anguria. Acta Biol Hung 69(1):97–109. https://doi.org/10.1556/018.68.2018.1.8
Chung IM, Rekha K, Rajakumar G, Thiruvengadam M (2018c) Elicitation of silver nanoparticles enhanced the secondary metabolites and pharmacological activities in cell suspension cultures of bitter gourd. 3 Biotech 8(10):1–12. https://doi.org/10.1007/s13205-018-1439-0
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé* D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci C 57(5):779–795. https://doi.org/10.1007/s000180050041
Debnath B, Li M, Liu S, Pan T, Ma C, Qiu D (2020) Melatonin-mediate acid rain stress tolerance mechanism through alteration of transcriptional factors and secondary metabolites gene expression in tomato. Ecotoxicol Environ Saf 200:110720. https://doi.org/10.1016/j.ecoenv.2020.110720
Ebadollahi R, Jafarirad S, Kosari-Nasab M, Mahjouri S (2019) Effect of explant source, perlite nanoparticles and TiO(2)/perlite nanocomposites on phytochemical composition of metabolites in callus cultures of Hypericum perforatum. Sci Rep 9(1):12998. https://doi.org/10.1038/s41598-019-49504-3
Eguchi R, Ono N, Hirai Morita A, Katsuragi T, Nakamura S, Huang M, Altaf-Ul-Amin M, Kanaya S (2019) Classification of alkaloids according to the starting substances of their biosynthetic pathways using graph convolutional neural networks. BMC Bioinformatics 20(1):380. https://doi.org/10.1186/s12859-019-2963-6
Elmer W, White JC (2018) The Future of Nanotechnology in Plant Pathology. Annu Rev Phytopathol 56(1):111–133. https://doi.org/10.1146/annurev-phyto-080417-050108
Erb M, Kliebenstein DJ (2020) Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol 184(1):39–52. https://doi.org/10.1104/pp.20.00433
Faisal M, Saquib Q, Alatar AA, Al-Khedhairy AA, Ahmed M, Ansari SM, Alwathnani HA, Dwivedi S, Musarrat J, Praveen S (2016) Cobalt oxide nanoparticles aggravate DNA damage and cell death in eggplant via mitochondrial swelling and NO signaling pathway. Biol Res 49(1):20. https://doi.org/10.1186/s40659-016-0080-9
Fazal H, Abbasi BH, Ahmad N, Ali M (2016) Elicitation of Medicinally Important Antioxidant Secondary Metabolites with Silver and Gold Nanoparticles in Callus Cultures of Prunella vulgaris L. Appl Biochem Biotechnol 180(6):1076–1092. https://doi.org/10.1007/s12010-016-2153-1
Feng L, Xu N, Qu Q, Zhang Z, Ke M, Lu T, Qian H (2021a) Synergetic toxicity of silver nanoparticle and glyphosate on wheat (Triticum aestivum L.). Sci Total Environ 797:149200. https://doi.org/10.1016/j.scitotenv.2021a.149200
Feng Z, Ji S, Ping J, Cui D (2021b) Recent advances in metabolomics for studying heavy metal stress in plants. TrAC Trends Anal Chem 143:116402. https://doi.org/10.1016/j.trac.2021.116402
Fu PP, Xia Q, Hwang HM, Ray PC, Yu H (2014) Mechanisms of nanotoxicity: Generation of reactive oxygen species. J Food Drug Anal 22(1):64–75. https://doi.org/10.1016/j.jfda.2014.01.005
Ghanati F, Bakhtiarian S (2014) Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula Officinalis L (Asteraceae). Trop J Pharm Res 13(11):1783–1789. https://doi.org/10.4314/tjpr.v13i11.2
Ghasemi B, Hosseini R, Dehghan Nayeri F (2015) Effects of cobalt nanoparticles on artemisinin production and gene expression in Artemisia annua. Turk J Botany 39(5):769–777. https://doi.org/10.3906/bot-1410-9
Ghorbanpour M, Hatami M, Hatami M (2015) Activating antioxidant enzymes, hyoscyamine and scopolamine biosynthesis of Hyoscyamus Niger L. Plants with nano-sized titanium dioxide and bulk Application. Acta Agric Slov 105(1):23–32. https://doi.org/10.14720/aas.2015.105.1.03
Golkar P, Moradi M, Garousi GA (2019) Elicitation of Stevia Glycosides Using Salicylic Acid and Silver Nanoparticles Under Callus Culture. Sugar Tech 21(4):569–577. https://doi.org/10.1007/s12355-018-0655-6
Gui X, Deng Y, Rui Y, Gao B, Luo W, Chen S, Van Nhan L, Li X, Liu S, Han Y, Liu L, Xing B (2015) Response difference of transgenic and conventional rice (Oryza sativa) to nanoparticles (γFe2O3). Environ Sci Pollut Res 22(22):17716–17723. https://doi.org/10.1007/s11356-015-4976-7
Hadi Soltanabad M, Bagherieh-Najjar MB, Mianabadi M (2020) Carnosic Acid Content Increased by Silver Nanoparticle Treatment in Rosemary (Rosmarinus officinalis L.). Appl Biochem Biotechnol 191(2):482–495. https://doi.org/10.1007/s12010-019-03193-w
Hao Y, Yu F, Lv R, Ma C, Zhang Z, Rui Y, Liu L, Cao W, Xing B (2016) Carbon Nanotubes Filled with Different Ferromagnetic Alloys Affect the Growth and Development of Rice Seedlings by Changing the C: N Ratio and Plant Hormones Concentrations. PLoS ONE 11(6):e0157264. https://doi.org/10.1371/journal.pone.0157264
Hedayati A, Hosseini B, Palazon J, Maleki R (2020) Improved tropane alkaloid production and changes in gene expression in hairy root cultures of two Hyoscyamus species elicited by silicon dioxide nanoparticles. Plant Physiol Biochem 155:416–428. https://doi.org/10.1016/j.plaphy.2020.07.029
Hedayati A, Naseri F, Nourozi E, Hosseini B, Honari H, Hemmaty S (2022) Response of Saponaria officinalis L. hairy roots to the application of TiO2 nanoparticles in terms of production of valuable polyphenolic compounds and SO6 protein. Plant Physiol Biochem 178:80–92. https://doi.org/10.1016/j.plaphy.2022.03.001
Hossain Z, Mustafa G, Komatsu S (2015) Plant responses to nanoparticle stress. Int J Mol Sci 16(11):26644–26653. https://doi.org/10.3390/ijms161125980
Hu X, Zhou Q (2015) Novel hydrated graphene ribbon unexpectedly promotes aged seed germination and root differentiation. Sci Rep 4(1):3782. https://doi.org/10.1038/srep03782
Huang Y, Adeleye AS, Zhao L, Minakova AS, Anumol T, Keller AA (2019) Antioxidant response of cucumber (Cucumis sativus) exposed to nano copper pesticide: Quantitative determination via LC-MS/MS. Food Chem 270:47–52. https://doi.org/10.1016/j.foodchem.2018.07.069
Jalil SU, Ansari MI (2019) Nanoparticles and abiotic stress tolerance in plants: synthesis, action, and signaling mechanisms. In Plant Signaling Molecules: Role and Regulation under Stressful Environments. Elsevier Inc. https://doi.org/10.1016/B978-0-12-816451-8.00034-4
Jamshidi M, Ghanati F (2017) Taxanes content and cytotoxicity of hazel cells extract after elicitation with silver nanoparticles. Plant Physiol Biochem 110:178–184. https://doi.org/10.1016/j.plaphy.2016.04.026
Jan R, Asaf S, Numan M, Lubna K-M (2021) Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 11(5):968. https://doi.org/10.3390/agronomy11050968
Jasim B, Thomas R, Mathew J, Radhakrishnan EK (2017) Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm J 25(3):443-447 https://doi.org/10.1016/j.jsps.2016.09.012
Javed R, Usman M, Yücesan B, Zia M, Gürel E (2017) Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol Biochem 110:94–99. https://doi.org/10.1016/j.plaphy.2016.05.032
Jiang HS, Yin LY, Ren NN, Zhao ST, Li Z, Zhi Y, Shao H, Li W, Gontero B (2017) Silver nanoparticles induced reactive oxygen species via photosynthetic energy transport imbalance in an aquatic plant. Nanotoxicology 11(2):157–167. https://doi.org/10.1080/17435390.2017.1278802
Juárez-Maldonado A, Ortega-Ortíz H, Morales-Díaz AB, González-Morales S, Morelos-Moreno Á, Cabrera-De la Fuente M, Sandoval-Rangel A, Cadenas-Pliego G, Benavides-Mendoza A (2019) Nanoparticles and Nanomaterials as Plant Biostimulants. Int J Mol Sci 20(1):162. https://doi.org/10.3390/ijms20010162
Kahila MMH, Najy AM, Rahaie M, Mir-Derikvand M (2018) Effect of nanoparticle treatment on expression of a key gene involved in thymoquinone biosynthetic pathway in Nigella sativa L. Nat Prod Res 32(15):1858–1862. https://doi.org/10.1080/14786419.2017.1405398
Kalisz A, Húska D, Jurkow R, Dvořák M, Klejdus B, Caruso G, Sękara A (2021) Nanoparticles of cerium, iron, and silicon oxides change the metabolism of phenols and flavonoids in butterhead lettuce and sweet pepper seedlings. Environ Sci Nano 8(7):1945–1959. https://doi.org/10.1039/d1en00262g
Kamalizadeh M, Bihamta M, Zarei A (2019) Drought stress and TiO2 nanoparticles affect the composition of different active compounds in the Moldavian dragonhead plant. Acta Physiol Plant 41(2):21. https://doi.org/10.1007/s11738-019-2814-0
Kang H, Hwang YG, Lee TG, Jin CR, Cho CH, Jeong HY, Kim DO (2016) Use of gold nanoparticle fertilizer enhances the ginsenoside contents and anti-inflammatory effects of red ginseng. J Microbiol Biotechnol 26(10):1668–1674. https://doi.org/10.4014/jmb.1604.04034
Kang W, Li X, Sun A, Yu F, Hu X (2019) Study of the Persistence of the Phytotoxicity Induced by Graphene Oxide Quantum Dots and of the Specific Molecular Mechanisms by Integrating Omics and Regular Analyses. Environ Sci Technol 53(7):3791–3801. https://doi.org/10.1021/acs.est.8b06023
Karakaş Ö (2020) Effect of Silver Nanoparticles on Production of Indole Alkaloids in Isatis constricta. Iran J Sci Technol Trans A Sci 44(3):621–627. https://doi.org/10.1007/s40995-020-00878-4
Karimzadeh F, Haddad R, Garoosi G, Khademian R (2019) Effects of Nanoparticles on Activity of Lignan Biosynthesis Enzymes in Cell Suspension Culture of Linum usitatissimum L. Russ J Plant Physiol 66(5):756–762. https://doi.org/10.1134/S1021443719050078
Kaveh R, Li YS, Ranjbar S, Tehrani R, Brueck CL, Van Aken B (2013) Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol 47(18):10637–10644. https://doi.org/10.1021/es402209w
Ke M, Qu Q, Peijnenburg WJGM, Li X, Zhang M, Zhang Z, Lu T, Pan X, Qian H (2018) Phytotoxic effects of silver nanoparticles and silver ions to Arabidopsis thaliana as revealed by analysis of molecular responses and of metabolic pathways. Sci Total Environ 644:1070–1079. https://doi.org/10.1016/j.scitotenv.2018.07.061
Khare S, Singh NB, Singh A, Hussain I, Niharika K, Yadav V, Bano C, Yadav RK, Amist N (2020) Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J Plant Biol 63(3):203–216. https://doi.org/10.1007/s12374-020-09245-7
Kim T, Song B, Cho KS, Lee I-S (2020) Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int J Mol Sci 21(6):2187. https://doi.org/10.3390/ijms21062187
Kiokias S, Proestos C, Oreopoulou V (2020) Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 9(4):534. https://doi.org/10.3390/foods9040534
Kruszka D, Sawikowska A, Kamalabai Selvakesavan R, Krajewski P, Kachlicki P, Franklin G (2020) Silver nanoparticles affect phenolic and phytoalexin composition of Arabidopsis thaliana. Sci Total Environ 716:135361. https://doi.org/10.1016/j.scitotenv.2019.135361
Kruszka D, Selvakesavan RK, Kachlicki P, Franklin G (2022) Untargeted metabolomics analysis reveals the elicitation of important secondary metabolites upon treatment with various metal and metal oxide nanoparticles in Hypericum perforatum L. cell suspension cultures. Ind Crops Prod 178:114561. https://doi.org/10.1016/j.indcrop.2022.114561
Lafmejani ZN, Jafari AA, Moradi P, Moghadam AL (2018) Impact of foliar application of copper sulphate and copper nanoparticles on some morpho-physiological traits and essential oil composition of peppermint (Mentha piperita L.). Herba Pol 64(2):13–24. https://doi.org/10.2478/hepo-2018-0006
Lala S (2020) Enhancement of secondary metabolites in Bacopa monnieri (L.) Pennell plants treated with copper‐based nanoparticles in vivo. IET Nanobiotechnology 14(1):78–85. https://doi.org/10.1049/iet-nbt.2019.0124
Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D (2006) Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic Biol Med 40(8):1369–1376. https://doi.org/10.1016/j.freeradbiomed.2005.12.006
Landa P, Prerostova S, Petrova S, Knirsch V, Vankova R, Vanek T (2015) The Transcriptomic Response of Arabidopsis thaliana to Zinc Oxide: A Comparison of the Impact of Nanoparticle, Bulk, and Ionic Zinc. Environ Sci Technol 49(24):14537–14545. https://doi.org/10.1021/acs.est.5b03330
Landa P, Vankova R, Andrlova J, Hodek J, Marsik P, Storchova H, White JC, Vanek T (2012) Nanoparticle-specific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J Hazard Mater 241–242(September):55–62. https://doi.org/10.1016/j.jhazmat.2012.08.059
Li S, Liu J, Wang Y, Gao Y, Zhang Z, Xu J, Xing G (2021a) Comparative physiological and metabolomic analyses revealed that foliar spraying with zinc oxide and silica nanoparticles modulates metabolite profiles in cucumber (Cucumis sativus L.). Food Energy Secur 10(1):1–13. https://doi.org/10.1002/fes3.269
Li X, Mu L, Hu X (2018a) Integrating proteomics, metabolomics and typical analysis to investigate the uptake and oxidative stress of graphene oxide and polycyclic aromatic hydrocarbons. Environ Sci Nano 5(1):115–129. https://doi.org/10.1039/C7EN00803A
Li X, Mu L, Li D, Ouyang S, He C, Hu X (2018b) Effects of the size and oxidation of graphene oxide on crop quality and specific molecular pathways. Carbon N Y 140:352–361. https://doi.org/10.1016/j.carbon.2018.08.063
Li X, Peng T, Mu L, Hu X (2019) Phytotoxicity induced by engineered nanomaterials as explored by metabolomics: Perspectives and challenges. Ecotoxicol Environ Saf 184(July):109602. https://doi.org/10.1016/j.ecoenv.2019.109602
Li Y, Liang L, Li W, Ashraf U, Ma L, Tang X, Pan S, Tian H, Mo Z (2021b) ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. J Nanobiotechnology 19(1):1–19. https://doi.org/10.1186/s12951-021-00820-9
López-Vargas ER, Ortega-Ortíz H, Cadenas-Pliego G, De Alba Romenus K, Cabrera de la Fuente M, Benavides-Mendoza A, Juárez-Maldonado A (2018) Foliar Application of Copper Nanoparticles Increases the Fruit Quality and the Content of Bioactive Compounds in Tomatoes. Appl Sci 8(7):1020. https://doi.org/10.3390/app8071020
Ma C, Liu H, Guo H, Musante C, Coskun SH, Nelson BC, White JC, Xing B, Dhankher OP (2016) Defense mechanisms and nutrient displacement in Arabidopsis thaliana upon exposure to CeO2 and In2O3 nanoparticles. Environ Sci Nano 3(6):1369–1379. https://doi.org/10.1039/C6EN00189K
Ma YJ, Xia J, Wang Y, Wang JW (2020) Stimulation of tanshinone production in Salvia miltiorrhiza hairy roots by β -cyclodextrin-coated silver nanoparticles. Sustain Chem Pharm 18:100271. https://doi.org/10.1016/j.scp.2020.100271
Mahajan M, Kuiry R, Pal PK (2020) Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J Appl Res Med Aromat Plants 18:100255. https://doi.org/10.1016/j.jarmap.2020.100255
Maina S, Misinzo G, Bakari G, Kim H-Y (2020) Human, Animal and Plant Health Benefits of Glucosinolates and Strategies for Enhanced Bioactivity: A Systematic Review. Mol. 25(16):3682. https://doi.org/10.3390/molecules25163682
Marioni F, Bertoli A, Pistelli L (2008) A straightforward procedure to biosynthesise melatonin using freshly chopped Achillea millefolium L. as reagent. Phytochem Lett 1(2):107–110. https://doi.org/10.1016/j.phytol.2008.06.001
Marslin G, Sheeba CJ, Franklin G (2017) Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front Plant Sci 8(May):832. https://doi.org/10.3389/fpls.2017.00832
McGehee DL, Lahiani MH, Irin F, Green MJ, Khodakovskaya MV (2017) Multiwalled Carbon Nanotubes Dramatically Affect the Fruit Metabolome of Exposed Tomato Plants. ACS Appl Mater Interfaces 9(38):32430–32435. https://doi.org/10.1021/acsami.7b10511
Mena P, Galindo A, Collado-González J, Ondoño S, García-Viguera C, Ferreres F, Torrecillas A, Gil-Izquierdo A (2013) Sustained deficit irrigation affects the colour and phytochemical characteristics of pomegranate juice. J Sci Food Agric 93(8):1922–1927. https://doi.org/10.1002/jsfa.5991
Miao Y, Xu J, Shen Y, Chen L, Bian Y, Hu Y, Zhou W, Zheng F, Man N, Shen Y, Zhang Y, Wang M, Wen L (2014) Nanoparticle as Signaling Protein Mimic: Robust Structural and Functional Modulation of CaMKII upon Specific Binding to Fullerene C60 Nanocrystals. ACS Nano 8(6):6131–6144. https://doi.org/10.1021/nn501495a
Mirzajani F, Askari H, Hamzelou S, Schober Y, Römpp A, Ghassempour A, Spengler B (2014) Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicol Environ Saf 108:335–339. https://doi.org/10.1016/j.ecoenv.2014.07.013
Modarresi M, Chahardoli A, Karimi N, Chahardoli S (2020) Variations of glaucine, quercetin and kaempferol contents in Nigella arvensis against Al2O3, NiO, and TiO2 nanoparticles. Heliyon 6(6):e04265. https://doi.org/10.1016/j.heliyon.2020.e04265
Moharrami F, Hosseini BB, Sharafi A, Farjaminezhad M (2017) Enhanced production of hyoscyamine and scopolamine from genetically transformed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. In Vitro Cell Dev Biol Plant 53(2):104–111. https://doi.org/10.1007/s11627-017-9802-0
Moola AK, Senthil Kumar T, Ranjitha Kumari BD (2021) Enhancement of Celastrol compound by silver nanoparticles and acetosyringone in Celastrus paniculatus Willd. through adventitious and hairy root culture. J Plant Biochem Biotechnol 31:429–434. https://doi.org/10.1007/s13562-021-00676-y
Mosquera-Sánchez LP, Arciniegas-Grijalba PA, Patiño-Portela MC, Guerra–Sierra BE, Muñoz-Florez JE, Rodríguez-Páez JE (2020) Antifungal effect of zinc oxide nanoparticles (ZnO-NPs) on Colletotrichum sp., causal agent of anthracnose in coffee crops. Biocatal Agric Biotechnol 25(March):101579. https://doi.org/10.1016/j.bcab.2020.101579
Nazir S, Jan H, Zaman G, Khan T, Ashraf H, Meer B, Zia M, Drouet S, Hano C, Abbasi BH (2021) Copper oxide (CuO) and manganese oxide (MnO) nanoparticles induced biomass accumulation, antioxidants biosynthesis and abiotic elicitation of bioactive compounds in callus cultures of Ocimum basilicum (Thai basil). Artif Cells, Nanomedicine, Biotechnol 49(1):626–634. https://doi.org/10.1080/21691401.2021.1984935
Nhan VL, Ma C, Rui Y, Liu S, Li X, Xing B, Liu L (2015) Phytotoxic mechanism of nanoparticles: Destruction of chloroplasts and vascular bundles and alteration of nutrient absorption. Sci Rep 5(1):1–13. https://doi.org/10.1038/srep11618
Nobahar A, Carlier JD, Miguel MG, Costa MC (2021) A review of plant metabolites with metal interaction capacity: a green approach for industrial applications. Biometals 34(4):761–793. https://doi.org/10.1007/s10534-021-00315-y
Nourozi E, Hosseini B, Maleki R, Abdollahi Mandoulakani B (2019a) Iron oxide nanoparticles: a novel elicitor to enhance anticancer flavonoid production and gene expression in Dracocephalum kotschyi hairy-root cultures. J Sci Food Agric 99(14):6418–6430. https://doi.org/10.1002/jsfa.9921
Nourozi E, Hosseini B, Maleki R, Mandoulakani BA (2019b) Pharmaceutical important phenolic compounds overproduction and gene expression analysis in Dracocephalum kotschyi hairy roots elicited by SiO2 nanoparticles. Ind Crops Prod 133:435–446. https://doi.org/10.1016/j.indcrop.2019.03.053
Oloumi H, Soltaninejad R, Baghizadeh A (2015) The comparative effects of nano and bulk size particles of CuO and ZnO on glycyrrhizin and phenolic compounds contents in Glycyrrhiza glabra L. seedlings. Indian J Plant Physiol 20(2):157–161. https://doi.org/10.1007/s40502-015-0143-x
Ouyang S, Hu X, Zhou Q (2015) Envelopment-Internalization Synergistic Effects and Metabolic Mechanisms of Graphene Oxide on Single-Cell Chlorella vulgaris Are Dependent on the Nanomaterial Particle Size. ACS Appl Mater Interfaces 7(32):18104–18112. https://doi.org/10.1021/acsami.5b05328
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47–e47. https://doi.org/10.1017/jns.2016.41
Panda KK, Achary VMM, Krishnaveni R, Padhi BK, Sarangi SN, Sahu SN, Panda BB (2011) In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol Vitr 25(5):1097–1105. https://doi.org/10.1016/j.tiv.2011.03.008
Pang Z, Chen J, Wang T, Gao C, Li Z, Guo L, Xu J, Cheng Y (2021) Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front Plant Sci 12:621276. https://doi.org/10.3389/fpls.2021.621276
Piasecka A, Jedrzejczak-Rey N, Bednarek P (2015) Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol 206:948–964. https://doi.org/10.1111/nph.13325
Poveda J, Eugui D, Velasco P (2020) Natural control of plant pathogens through glucosinolates: an effective strategy against fungi and oomycetes. Phytochem Rev 19(4):1045–1059. https://doi.org/10.1007/s11101-020-09699-0
Qi P, Li J, Gao S, Yuan Y, Sun Y, Liu N, Li Y, Wang G, Chen L, Shi J (2020) Network Pharmacology-Based and Experimental Identification of the Effects of Quercetin on Alzheimer’s Disease. Front Aging Neurosci 12:589588. https://doi.org/10.3389/fnagi.2020.589588
Rajput VD, Minkina T, Kumari A, Harish, Singh VK, Verma KK, Mandzhieva S, Sushkova S, Srivastava S, Keswani C (2021) Coping with the Challenges of Abiotic Stress in Plants: New Dimensions in the Field Application of Nanoparticles. Plants 10(6):1221. https://doi.org/10.3390/plants10061221
Ramezannezhad R, Aghdasi M, Fatemi M (2019) Enhanced production of cichoric acid in cell suspension culture of Echinacea purpurea by silver nanoparticle elicitation. Plant Cell, Tissue Organ Cult 139(2):261–273. https://doi.org/10.1007/s11240-019-01678-4
Ranjan A, Rajput VD, Minkina T, Bauer T, Chauhan A, Jindal T (2021) Nanoparticles induced stress and toxicity in plants. Environ Nanotechnology, Monit Manag 15:100457. https://doi.org/10.1016/j.enmm.2021.100457
Rico CM, Lee SC, Rubenecia R, Mukherjee A, Hong J, Peralta-Videa JR, Gardea-Torresdey JL (2014) Cerium Oxide Nanoparticles Impact Yield and Modify Nutritional Parameters in Wheat ( Triticum aestivum L.). J Agric Food Chem 62(40):9669–9675. https://doi.org/10.1021/jf503526r
de Rivero-Montejo S, J, Vargas-Hernandez M, Torres-Pacheco I, (2021) Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agric 11(2):1–16. https://doi.org/10.3390/agriculture11020134
Sadak MS, Bakry BA (2020) Zinc-oxide and nano ZnO oxide effects on growth, some biochemical aspects, yield quantity, and quality of flax (Linum uitatissimum L.) in absence and presence of compost under sandy soil. Bull Natl Res Cent 44(1):98. https://doi.org/10.1186/s42269-020-00348-2
Salehi H, Chehregani A, Lucini L, Majd A, Gholami M (2018) Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaseolus vulgaris L. under soil or foliar application. Sci Total Environ 616–617:1540–1551. https://doi.org/10.1016/j.scitotenv.2017.10.159
Salehi H, Miras-Moreno B, Chehregani Rad A, Pii Y, Mimmo T, Cesco S, Lucini L (2020) Relatively Low Dosages of CeO2 Nanoparticles in the Solid Medium Induce Adjustments in the Secondary Metabolism and Ionomic Balance of Bean (Phaseolus vulgaris L.) Roots and Leaves. J Agric Food Chem 68(1):67–76. https://doi.org/10.1021/acs.jafc.9b05107
Samadi S, Saharkhiz MJ, Azizi M, Samiei L, Karami A, Ghorbanpour M (2021) Single-wall carbon nano tubes (SWCNTs) penetrate Thymus daenensis Celak. plant cells and increase secondary metabolite accumulation in vitro. Ind Crops Prod 165:113424. https://doi.org/10.1016/j.indcrop.2021.113424
Santner A, Calderon-Villalobos LIA, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5(5):301–307. https://doi.org/10.1038/nchembio.165
Shabbir A, Khan MMA, Ahmad B, Sadiq Y, Jaleel H, Uddin M (2019) Efficacy of TiO2 nanoparticles in enhancing the photosynthesis, essential oil and khusimol biosynthesis in Vetiveria zizanioides L. Nash. Photosynthetica 57(2):599–606. https://doi.org/10.32615/ps.2019.071
Shakeran Z, Keyhanfar M, Asghari G, Ghanadian M (2015) Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel. TURKISH J Biol 39(1):111–118. https://doi.org/10.3906/biy-1405-25
Shakya P, Marslin G, Siram K, Beerhues L, Franklin G (2019) Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J Pharm Pharmacol 71(1):70–82. https://doi.org/10.1111/jphp.12743
Shehzad M, aamir, Khan MA, Ali A, Mohammad S, Noureldeen A, Darwish H, Ali A, Ahmad A, Khan T, Khan RS, (2021) Interactive effects of zinc oxide nano particles and different light regimes on growth and silymarin biosynthesis in callus cultures of Silybum marianum L. Artif Cells, Nanomedicine, Biotechnol 49(1):523–535. https://doi.org/10.1080/21691401.2021.1946069
Shoja AA, Çirak C, Ganjeali A, Cheniany M (2022) Stimulation of phenolic compounds accumulation and antioxidant activity in in vitro culture of Salvia tebesana Bunge in response to nano-TiO2 and methyl jasmonate elicitors. Plant Cell, Tissue Organ Cult 149:423–440. https://doi.org/10.1007/s11240-022-02251-2
Singh R, Singh DP, Gupta P, Jain P (2018) Sanchita, Mishra T, Kumar A, Dhawan SS, Shirke PA (2019) Nanoparticles alter the withanolide biosynthesis and carbohydrate metabolism in Withania somnifera (Dunal). Ind Crops Prod 127:94–109. https://doi.org/10.1016/j.indcrop.2018.10.049
Soares C, Branco-Neves S, de Sousa A, Azenha M, Cunha A, Pereira R, Fidalgo F (2018) SiO2 nanomaterial as a tool to improve Hordeum vulgare L. tolerance to nano-NiO stress. Sci Total Environ 622–623:517–525. https://doi.org/10.1016/j.scitotenv.2017.12.002
Sosan A, Svistunenko D, Straltsova D, Tsiurkina K, Smolich I, Lawson T, Subramaniam S, Golovko V, Anderson D, Sokolik A, Colbeck I, Demidchik V (2016) Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. Plant J 85(2):245–257. https://doi.org/10.1111/tpj.13105
Stevenson PC (2020) For antagonists and mutualists: the paradox of insect toxic secondary metabolites in nectar and pollen. Phytochem Rev 19(3):603–614. https://doi.org/10.1007/s11101-019-09642-y
Sun L, Song F, Guo J, Zhu X, Liu S, Liu F, Li X (2020) Nano-ZnO-Induced Drought Tolerance Is Associated with Melatonin Synthesis and Metabolism in Maize. Int J Mol Sci 21(3):782. https://doi.org/10.3390/ijms21030782
Sun TY, Gottschalk F, Hungerbühler K, Nowack B (2014) Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ Pollut 185:69–76. https://doi.org/10.1016/j.envpol.2013.10.004
Syu Y, yu, Hung JH, Chen JC, Chuang H wen, (2014) Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem 83:57–64. https://doi.org/10.1016/j.plaphy.2014.07.010
Taghizadeh M, Nasibi F, Manouchehri Kalantari K, Mohseni-Moghadam M (2021) Modification of phytochemical production and antioxidant activity of Dracocephalum kotschyi cells by exposure to static magnetic field and magnetite nanoparticles. Plant Cell, Tissue Organ Cult 147(2):365–377. https://doi.org/10.1007/s11240-021-02129-9
Talankova-Sereda TE, Liapina KV, Shkopinskij EA, Ustinov AI, Kovalyova AV, Dulnev PG, Kucenko NI (2016) The Influence of Cu и Co Nanoparticles on Growth Characteristics and Biochemical Structure of Mentha Longifolia In Vitro. In: Fesenko O, Yatsenko L (eds) Nanophysics, Nanophotonics, Surface Studies, and Applications. Springer Proceedings in Physics. 183:427-436. Springer, Cham. https://doi.org/10.1007/978-3-319-30737-4_36
Thakur M, Bhattacharya S, Khosla PK, Puri S (2019) Improving production of plant secondary metabolites through biotic and abiotic elicitation. J Appl Res Med Aromat Plants 12:1–12. https://doi.org/10.1016/j.jarmap.2018.11.004
Thiruvengadam M, Gurunathan S, Chung IM (2015) Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma 252(4):1031–1046. https://doi.org/10.1007/s00709-014-0738-5
Tripathi DK, Singh S, Singh S, Srivastava PK, Singh VP, Singh S, Prasad SM, Singh PK, Dubey NK, Pandey AC, Chauhan DK (2017) Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol Biochem 110:167–177. https://doi.org/10.1016/j.plaphy.2016.06.015
Tusevski O, Krstikj M, Stanoeva JP, Stefova M, Gadzovska Simic S (2018) Phenolic profile and biological activity of Hypericum perforatum L.: Can roots be considered as a new source of natural compounds? South African J Bot 117:301–310. https://doi.org/10.1016/j.sajb.2018.05.030
Tuteja N, Mahajan S (2007) Calcium Signaling Network in Plants. Plant Signal Behav 2(2):79–85. https://doi.org/10.4161/psb.2.2.4176
Vahide P, Negar K, Hajati Razieh J (2021) Bio nanoparticles as elicitors increase accumulation of betulin and betulinic acid in callus cultures. South African J Bot 141:431–439. https://doi.org/10.1016/j.sajb.2021.05.005
Van Aken B (2015) Gene expression changes in plants and microorganisms exposed to nanomaterials. Curr Opin Biotechnol 33:206–219. https://doi.org/10.1016/j.copbio.2015.03.005
Van Nguyen D, Nguyen HM, Le NT, Nguyen KH, Nguyen HT, Le HM, Nguyen AT, Dinh NTT, Hoang SA, Van Ha C (2021) Copper Nanoparticle Application Enhances Plant Growth and Grain Yield in Maize Under Drought Stress Conditions. J Plant Growth Regul 41:364–375. https://doi.org/10.1007/s00344-021-10301-w
Vankova R, Landa P, Podlipna R, Dobrev PI, Prerostova S, Langhansova L, Gaudinova A, Motkova K, Knirsch V, Vanek T (2017) ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Sci Total Environ 593–594:535–542. https://doi.org/10.1016/j.scitotenv.2017.03.160
Vannini C, Domingo G, Onelli E, Prinsi B, Marsoni M, Espen L, Bracale M (2013) Morphological and Proteomic Responses of Eruca sativa Exposed to Silver Nanoparticles or Silver Nitrate. PLoS One 8(7). https://doi.org/10.1371/journal.pone.0068752
Večeřová K, Večeřa Z, Dočekal B, Oravec M, Pompeiano A, Tříska J, Urban O (2016) Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environ Pollut 218:207–218. https://doi.org/10.1016/j.envpol.2016.05.013
Velingkar VS, Gupta GL, Hegde NB (2017) A current update on phytochemistry, pharmacology and herb–drug interactions of Hypericum perforatum. Phytochem Rev 16(4):725–744. https://doi.org/10.1007/s11101-017-9503-7
Wang B, Guan C, Fu Q (2021) The traditional uses, secondary metabolites, and pharmacology of Lycopodium species. Phytochem Rev 21:1–79. https://doi.org/10.1007/s11101-021-09746-4
Wang Y, Lin Y, Xu Y, Yin Y, Guo H, Du W (2019) Divergence in response of lettuce ( var. ramosa Hort .) to copper oxide nanoparticles/microparticles as potential agricultural fertilizer. Environ Pollut Bioavailab 31(1):80–84. https://doi.org/10.1080/26395940.2019.1578187
Wu B, Zhu L, Le XC (2017) Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.). Environ Pollut 230:302–310. https://doi.org/10.1016/j.envpol.2017.06.062
Wu X, Huang H, Childs H, Wu Y, Yu L, Pehrsson PR (2021) Glucosinolates in Brassica Vegetables: Characterization and Factors That Influence Distribution, Content, and Intake. Annu Rev Food Sci Technol 12(1):485–511. https://doi.org/10.1146/annurev-food-070620-025744
Xu W-J, Li R-J, Quasie O, Yang M-H, Kong L-Y, Luo J (2016) Polyprenylated Tetraoxygenated Xanthones from the Roots of Hypericum monogynum and Their Neuroprotective Activities. J Nat Prod 79(8):1971–1981. https://doi.org/10.1021/acs.jnatprod.6b00251
Yazdanian E, Golkar P, Vahabi MR, Taghizadeh M (2021) Elicitation Effects on Some Secondary Metabolites and Antioxidant Activity in Callus Cultures of Allium jesdianum Boiss. & Buhse.: Methyl Jasmonate and Putrescine. Appl Biochem Biotechnol 194:601–619. https://doi.org/10.1007/s12010-021-03643-4
Yuan P, Zhou Q, Hu X (2018) The Phases of WS 2 Nanosheets Influence Uptake, Oxidative Stress, Lipid Peroxidation, Membrane Damage, and Metabolism in Algae. Environ Sci Technol 52(22):13543–13552. https://doi.org/10.1021/acs.est.8b04444
Zahedi SM, Hosseini MS, Daneshvar Hakimi Meybodi N, Peijnenburg W (2021) Mitigation of the effect of drought on growth and yield of pomegranates by foliar spraying of different sizes of selenium nanoparticles. J Sci Food Agric 101(12):5202–5213. https://doi.org/10.1002/jsfa.11167
Zahir A, Nadeem M, Ahmad W, Giglioli-Guivarc’h N, Hano C, Abbasi BH, (2019) Chemogenic silver nanoparticles enhance lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Plant Cell, Tissue Organ Cult 136(3):589–596. https://doi.org/10.1007/s11240-018-01539-6
Zhang B, Zheng LP, Wang JW (2012) Nitric oxide elicitation for secondary metabolite production in cultured plant cells. Appl Microbiol Biotechnol 93(2):455–466. https://doi.org/10.1007/s00253-011-3658-8
Zhang B, Zheng LP, Yi Li W, Wen Wang J (2013) Stimulation of Artemisinin Production in Artemisia annua Hairy Roots by Ag-SiO2 Core-shell Nanoparticles. Curr Nanosci 9(3):363–370. https://doi.org/10.2174/1573413711309030012
Zhang CL, Jiang HS, Gu SP, Zhou XH, Lu ZW, Kang XH, Yin L, Huang J (2019a) Combination analysis of the physiology and transcriptome provides insights into the mechanism of silver nanoparticles phytotoxicity. Environ Pollut 252:1539–1549. https://doi.org/10.1016/j.envpol.2019.06.032
Zhang H, Du W, Peralta-Videa JR, Gardea-Torresdey JL, White JC, Keller A, Guo H, Ji R, Zhao L (2018) Metabolomics Reveals How Cucumber ( Cucumis sativus ) Reprograms Metabolites To Cope with Silver Ions and Silver Nanoparticle-Induced Oxidative Stress. Environ Sci Technol 52(14):8016–8026. https://doi.org/10.1021/acs.est.8b02440
Zhang H, Lu L, Zhao X, Zhao S, Gu X, Du W, Wei H, Ji R, Zhao L (2019b) Metabolomics Reveals the “invisible” Responses of Spinach Plants Exposed to CeO2 Nanoparticles. Environ Sci Technol 53(10):6007–6017. https://doi.org/10.1021/acs.est.9b00593
Zhao L, Hu J, Huang Y, Wang H, Adeleye A, Ortiz C, Keller AA (2017a) 1H NMR and GC–MS based metabolomics reveal nano-Cu altered cucumber (Cucumis sativus) fruit nutritional supply. Plant Physiol Biochem 110:138–146. https://doi.org/10.1016/j.plaphy.2016.02.010
Zhao L, Huang Y, Adeleye AS, Keller AA (2017b) Metabolomics Reveals Cu(OH)2 Nanopesticide-Activated Anti-oxidative Pathways and Decreased Beneficial Antioxidants in Spinach Leaves. Environ Sci Technol 51(17):10184–10194. https://doi.org/10.1021/acs.est.7b02163
Zhao L, Huang Y, Hu J, Zhou H, Adeleye AS, Keller AA (2016a) 1H NMR and GC-MS Based Metabolomics Reveal Defense and Detoxification Mechanism of Cucumber Plant under Nano-Cu Stress. Environ Sci Technol 50(4):2000–2010. https://doi.org/10.1021/acs.est.5b05011
Zhao L, Huang Y, Keller AA (2018a) Comparative Metabolic Response between Cucumber ( Cucumis sativus ) and Corn ( Zea mays ) to a Cu(OH) 2 Nanopesticide. J Agric Food Chem 66(26):6628–6636. https://doi.org/10.1021/acs.jafc.7b01306
Zhao L, Huang Y, Paglia K, Vaniya A, Wancewicz B, Keller AA (2018b) Metabolomics Reveals the Molecular Mechanisms of Copper Induced Cucumber Leaf ( Cucumis sativus) Senescence. Environ Sci Technol 52(12):7092–7100. https://doi.org/10.1021/acs.est.8b00742
Zhao L, Ortiz C, Adeleye AS, Hu Q, Zhou H, Huang Y, Keller AA (2016b) Metabolomics to Detect Response of Lettuce ( Lactuca sativa ) to Cu(OH) 2 Nanopesticides: Oxidative Stress Response and Detoxification Mechanisms. Environ Sci Technol 50(17):9697–9707. https://doi.org/10.1021/acs.est.6b02763
Zhao L, Zhang H, Wang J, Tian L, Li F, Liu S, Peralta-Videa JR, Gardea-Torresdey JL, White JC, Huang Y, Keller A, Ji R (2019) C60 Fullerols Enhance Copper Toxicity and Alter the Leaf Metabolite and Protein Profile in Cucumber. Environ Sci Technol 53(4):2171–2180. https://doi.org/10.1021/acs.est.8b06758
Acknowledgements
This work was supported by the National Science Centre (NCN), OPUS project Reg. No. 2016/21/B/NZ9/01980. RKS and DK are recipients of PhD and postdoctoral fellowships, respectively, from this project. DM and GF acknowledge the NANOPLANT project, which received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No. 856691. The authors thank Mr F. Gerin Danny, Kings College London, for his help in figure 6.3 drawing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Selvakesavan, R.K., Kruszka, D., Shakya, P., Mondal, D., Franklin, G. (2023). Impact of Nanomaterials on Plant Secondary Metabolism. In: Al-Khayri, J.M., Alnaddaf, L.M., Jain, S.M. (eds) Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications. Springer, Cham. https://doi.org/10.1007/978-3-031-20878-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-20878-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20877-5
Online ISBN: 978-3-031-20878-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)