Abstract
Macrophages are the most abundant and one of the most critical cells of tumor immunity. They provide a bridge between innate and adaptive immunity through releasing cytokines into the tumor microenvironment (TME). A number of interleukin (IL) cytokine family members is involved in shaping the final phenotype of macrophages toward either a classically-activated pro-inflammatory M1 state with anti-tumor activity or an alternatively-activated anti-inflammatory M2 state with pro-tumor activity. Shaping TME macrophages toward the M1 phenotype or recovering this phenotypic state may offer a promising therapeutic approach in patients with cancer. Here, we focus on the impact of macrophage-polarizing ILs on immune cells and IL-mediated cellular cross-interactions within the TME. The key aim of this review is to define therapeutic schedules for addressing ILs in cancer immunotherapy based on their multi-directional impacts in such a milieu. Gathering more knowledge on this area is also important for defining adverse effects related to cytokine therapy and addressing them for reinforcing the efficacy of immunotherapy against cancer.




Similar content being viewed by others
References
M. Najafi et al., The current knowledge concerning solid cancer and therapy. J. Biochem. Mol. Toxicol. 35 e22900 (2021)
K. Mortezaee, Normalization in tumor ecosystem: Opportunities and challenges. Cell Biol. Int. 45(10), 2017–2030 (2021)
K. Mortezaee, Organ tropism in solid tumor metastasis: an updated review. Future Oncol. 17(15), 1943–1961 (2021)
X. Niu et al., Sodium/glucose cotransporter 1-dependent metabolic alterations induce tamoxifen resistance in breast cancer by promoting macrophage M2 polarization. Cell Death Dis. 12(6), 1–15 (2021)
Y.-K. Huang et al., Macrophage spatial heterogeneity in gastric cancer defined by multiplex immunohistochemistry. Nat. Commun. 10(1), 1–15 (2019)
B. Farhood et al., Disruption of the redox balance with either oxidative or anti-oxidative overloading as a promising target for cancer therapy. J. Cell. Biochem. 120(1), 71–76 (2019)
H. Xu et al., The IL-33/ST2 axis affects tumor growth by regulating mitophagy in macrophages and reprogramming their polarization. Cancer Biol. Med. 18(1), 172 (2021)
D. Saha, R.L. Martuza, S.D. Rabkin, Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell 32(2): 253–267. e5 (2017)
R. Zhang et al., GPR30 knockdown weakens the capacity of CAF in promoting prostate cancer cell invasion via reducing macrophage infiltration and M2 polarization. J. Cell. Biochem. 122 doi: 10.1002/jcb.29938 (2021)
L. Sun et al., Activating a collaborative innate-adaptive immune response to control metastasis. Cancer Cell 39(10), 1361–1374 (2021)
Y. Peng et al., Tumor-associated macrophages as treatment targets in glioma. Brain Sci. Adv. 6(4), 306–323 (2020)
Q. Wen et al., Fusion cytokine IL-2-GMCSF enhances anticancer immune responses through promoting cell–cell interactions. J. Transl. Med. 14(1), 1–13 (2016)
D. Briukhovetska et al., Interleukins in cancer: from biology to therapy. Nature Rev. Cancer 21(8), 481–499 (2021)
K. Yan et al., Multi-omics analysis identifies FoxO1 as a regulator of macrophage function through metabolic reprogramming. Cell Death Dis. 11(9), 1–14 (2020)
M. Yang et al., ICAM-1 suppresses tumor metastasis by inhibiting macrophage M2 polarization through blockade of efferocytosis. Cell Death Dis. 6(6), e1780–e1780 (2015)
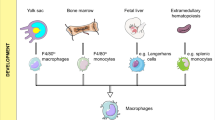
M. Guilliams, C.L. Scott, Does niche competition determine the origin of tissue-resident macrophages? Nat. Rev. Immunol. 17(7), 451–460 (2017)
S.A. Dick et al., Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci. Immunol. 7(67), eabf7777 (2022)
L.V. Ireland, A. Mielgo, Macrophages and fibroblasts, key players in cancer chemoresistance. Front. Cell Dev. Biol. 6, 131 (2018)
M.B. Buechler, W. Fu, S.J. Turley, Fibroblast-macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity 54(5), 903–915 (2021)
M.R. Spalinger et al., Loss of protein tyrosine phosphatase non-receptor type 2 reduces IL-4-driven alternative macrophage activation. Mucosal Immunol. 15(1), 74–83 (2022)
K. Kubota et al., CD163 + CD204 + tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci. Rep. 7(1), 1755 (2017)
D.H. Seo et al., Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci. Rep. 7(1), 851 (2017)
P. Sarode et al., Macrophage and tumor cell cross-talk is fundamental for lung tumor progression: we need to talk. Front. Oncol. 10, 324 (2020)
M. Akimoto et al., Soluble IL-33 receptor sST2 inhibits colorectal cancer malignant growth by modifying the tumour microenvironment. Nat. Commun. 7(1), 1–15 (2016)
Y. Pan et al., Tumor-associated macrophages in tumor immunity. Front. Immunol. 11, 3151 (2020)
C. Xu et al., Combination therapy with NHS-muIL12 and avelumab (anti-PD-L1) enhances antitumor efficacy in preclinical cancer models. Clin. Cancer Res. 23(19), 5869–5880 (2017)
J. Majidpoor, K. Mortezaee, Steps in metastasis: an updated review. Med. Oncol. 38(1), 1–17 (2021)
A.J. Boutilier, S.F. Elsawa, Macrophage polarization states in the tumor microenvironment. Int. J. Mol. Sci. 22(13), 6995 (2021)
J.P. Väyrynen et al., The prognostic role of macrophage polarization in the colorectal cancer microenvironment. Cancer Immunol. Res. 9(1), 8–19 (2021)
S. Zhu et al., Tumor-associated macrophages: role in tumorigenesis and immunotherapy implications. J. Cancer 12(1), 54 (2021)
C. Giordano et al., Combining magnetic resonance imaging with systemic monocyte evaluation for the implementation of GBM management. Int. J. Mol. Sci. 22(7), 3797 (2021)
M.-J. Lee et al., Results from a biomarker study to accompany a phase II trial of RRx-001 with reintroduced platinum-based chemotherapy in relapsed small cell carcinoma. Expert Opin. Investig. Drugs 30(2), 177–183 (2021)
F. Meng et al., Interaction between pancreatic cancer cells and tumor-associated macrophages promotes the invasion of pancreatic cancer cells and the differentiation and migration of macrophages. IUBMB life 66(12), 835–846 (2014)
T. Yamaguchi et al., Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer 19(4), 1052–1065 (2016)
A. Frafjord et al., Antibody combinations for optimized staining of macrophages in human lung tumours. Scand. J. Immunol. 92(1), e12889 (2020)
M. Zhang et al., A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 7(1), 1–16 (2014)
K. Mortezaee et al., Melatonin pretreatment enhances the homing of bone marrow-derived mesenchymal stem cells following transplantation in a rat model of liver fibrosis. Iran. Biomed. J. 20(4), 207 (2016)
K. Mortezaee et al., Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by CCl4. Cell Tissue Res. 369(2), 303–312 (2017)
B. Diskin et al., PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat. Immunol. 21(4), 442–454 (2020)
K. Mortezaee, Enriched cancer stem cells, dense stroma, and cold immunity: Interrelated events in pancreatic cancer. J. Biochem. Mol. Toxicol. 35(4), e22708 (2021)
K. Mortezaee, Myeloid-derived suppressor cells in cancer immunotherapy-clinical perspectives. Life Sc. 277, 119627 (2021)
S. Ostrand-Rosenberg et al. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Sem. Cancer Biol. 22(4), 275–281 (2012)
V.K. Deo, T. Kato, E.Y. Park, Virus-like particles displaying recombinant short-chain fragment region and interleukin 2 for targeting colon cancer tumors and attracting macrophages. J. Pharm. Sci. 105(5), 1614–1622 (2016)
M.E. Raeber et al., Interleukin-2 signals converge in a lymphoid–dendritic cell pathway that promotes anticancer immunity. Sci. Transl. Med. 12, 561 (2020)
C.M. Rollings et al., Interleukin-2 shapes the cytotoxic T cell proteome and immune environment–sensing programs. Sci. Signal. 11, 526 (2018)
X. Tong et al., The mechanism of chemokine receptor 9 internalization triggered by interleukin 2 and interleukin 4. Cell Mol. Immunol. 6(3), 181–189 (2009)
K.V. Bankaitis, B. Fingleton, Targeting IL4/IL4R for the treatment of epithelial cancer metastasis. Clin. Exp. Metastasis 32(8), 847–856 (2015)
X. Song et al., Possible roles of Interleukin-4 and-13 and their receptors in gastric and colon cancer. Int. J. Mol. Sci. 22(2), 727 (2021)
C.M. Wunderlich et al., Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat. Commun. 9(1), 1–16 (2018)
H. Xu et al., Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer 14(1), 1–13 (2014)
J.K. Singh et al., Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 15(4), 1–9 (2013)
D.S. Shouval et al., Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 40(5), 706–719 (2014)
R.A. Saxton et al., Structure-based decoupling of the pro-and anti-inflammatory functions of interleukin-10. Science 371, 6535 (2021)
C. Gorby et al., Engineered IL-10 variants elicit potent immunomodulatory effects at low ligand doses. Sci. Signal. 13(649), eabc0653 (2020)
K. Mortezaee, M. Najafi, Immune system in cancer radiotherapy: resistance mechanisms and therapy perspectives. Crit. Rev. Oncol./Hematol. 157, 103180 (2020)
S. Mondal et al., IL-12 p40 monomer is different from other IL-12 family members to selectively inhibit IL-12Rβ1 internalization and suppress EAE. Proc. Natl. Acad. Sci. USA 117(35), 21557–21567 (2020)
K.G. Nguyen et al., Localized interleukin-12 for cancer immunotherapy. Front. Immunol. 11, 575597(2020)
Y. Guo, W. Cao, Y. Zhu, Immunoregulatory functions of the IL-12 family of cytokines in antiviral systems. Viruses 11(9), 772 (2019)
M.F. Eissmann et al., IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat. Commun. 10(1), 1–16 (2019)
C. O’donnell et al., An antitumorigenic role for the IL-33 receptor, ST2L, in colon cancer. Br. J. Cancer 114(1), 37–43 (2016)
A. Masjedi et al., The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed. Pharmacother. 108, 1415–1424 (2018)
M.J. Waldner, S. Foersch, M.F. Neurath, Interleukin-6-a key regulator of colorectal cancer development. Int. J. Biol. Sci. 8(9), 1248 (2012)
M. Fang et al., IL33 promotes colon cancer cell stemness via JNK activation and macrophage recruitment. Cancer Res. 77(10), 2735–2745 (2017)
T. Vilsmaier et al., Influence of circulating tumour cells on production of IL-1α, IL-1β and IL-12 in sera of patients with primary diagnosis of breast cancer before treatment. Anticancer Res. 36(10), 5227–5236 (2016)
T. Zhang et al., Oncolytic tanapoxvirus expressing interleukin-2 is capable of inducing the regression of human melanoma tumors in the absence of T cells. Curr. Cancer Drug Targets 18(6), 577–591 (2018)
Z. Sun et al., A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8 + T-cell response and effective tumor control. Nat. Commun. 10(1), 1–12 (2019)
J.M. Santos et al., Adenoviral production of interleukin-2 at the tumor site removes the need for systemic postconditioning in adoptive cell therapy. Int. J. Cancer 141(7), 1458–1468 (2017)
A. Ahmed et al., Peripheral blood and tissue assessment highlights differential tumor-circulatory gradients of IL2 and MIF with prognostic significance in resectable pancreatic ductal adenocarcinoma. Oncoimmunology 10(1), 1962135 (2021)
J. Majidpoor, K. Mortezaee, Interleukin-2 therapy of cancer-clinical perspectives. Int. Immunopharmacol. 98, 107836 (2021)
G. Trinchieri, Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3(2), 133–146 (2003)
K.C. Hicks et al., Tumour-targeted interleukin-12 and entinostat combination therapy improves cancer survival by reprogramming the tumour immune cell landscape. Nat. Commun. 12(1), 1–18 (2021)
P.H. Thaker et al., A phase I trial of intraperitoneal GEN-1, an IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer, administered with pegylated liposomal doxorubicin in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancers: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 147(2), 283–290 (2017)
B. Mirlekar, Y. Pylayeva-Gupta, IL-12 Family Cytokines in cancer and immunotherapy. Cancers 13(2), 167 (2021)
W. Lasek, R. Zagożdżon, M. Jakobisiak, Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 63(5), 419–435 (2014)
K. Buscher et al., Natural variation of macrophage activation as disease-relevant phenotype predictive of inflammation and cancer survival. Nat. Commun. 8(1), 1–10 (2017)
E.A. Chiocca et al., Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Science Transl. Med. 11, 505 (2009)
N. Kirchhammer et al., Successful IL-12 cancer immunotherapy requires NK cell-derived CCL5 for anti-tumor DC-T cell crosstalk. BioRxiv, (2021)
S. Tugues et al., New insights into IL-12-mediated tumor suppression. Cell. Death Diff. 22(2), 237–246 (2015)
F. Castro et al., Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 9, 847 (2018)
Y. Che et al., Induction of systemic immune responses and reversion of immunosuppression in the tumor microenvironment by a therapeutic vaccine for cervical cancer. Cancer Immunol. Immunother. 69(12), 2651–2664 (2020)
A. Ryan et al., Targeting colon cancer cell NF-κB promotes an anti-tumour M1-like macrophage phenotype and inhibits peritoneal metastasis. Oncogene 34(12), 1563–1574 (2015)
R. Spolski, W.J. Leonard, Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 26, 57–79 (2008)
W.J. Leonard, R. Spolski, Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat. Rev. Immunol. 5(9), 688–698 (2005)
S. Deng et al., Targeting tumors with IL-21 reshapes the tumor microenvironment by proliferating PD-1intTim-3–CD8 + T cells. JCI Insight 5, 7 (2020)
Y. Wang et al., An IL-4/21 inverted cytokine receptor improving CAR-T cell potency in immunosuppressive solid-tumor microenvironment. Front. Immunol. 10, 1691 (2019)
M. Xu et al., Intratumoral delivery of IL-21 overcomes anti-Her2/Neu resistance through shifting tumor-associated macrophages from M2 to M1 phenotype. J. Immunol. 194(10), 4997–5006 (2015)
D. Xue et al., Next-generation cytokines for cancer immunotherapy. Antib. Ther. 4(2), 123–133 (2021)
V. Kannappan et al., Interleukin 21 inhibits cancer-mediated FOXP3 induction in naïve human CD4 T cells. Cancer Immunol. Immunother. 66(5), 637–645 (2017)
D. Hermans et al., Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8 + T cell stemness and antitumor immunity. Proc. Natl. Acad. Sci. USA 117(11), 6047–6055 (2020)
Y. Li et al., Targeting IL-21 to tumor-reactive T cells enhances memory T cell responses and anti-PD-1 antibody therapy. Nat. Commun. 12(1), 1–13 (2021)
K. Brandt et al., Interleukin-21 inhibits dendritic cell activation and maturation. Blood 102(12), 4090–4098 (2003)
S. Shen et al., Engineered IL-21 cytokine muteins fused to anti-PD-1 antibodies can improve CD8 + T cell function and anti-tumor immunity. Front. Immunol. 11, 832 (2020)
Y. Zhao et al., IL-21 is an accomplice of PD-L1 in the induction of PD-1-dependent Treg generation in head and neck cancer. Front. Oncol. 11, 1601 (2021)
G. Nappo et al., The immunosuppressive cytokine interleukin-4 increases the clonogenic potential of prostate stem-like cells by activation of STAT6 signalling. Oncogenesis 6(5), e342–e342 (2017)
J. Sheng et al., M2 macrophage-mediated interleukin-4 signalling induces myofibroblast phenotype during the progression of benign prostatic hyperplasia. Cell Death Dis. 9(7), 1–13 (2018)
S. Parveen et al., IL-4 receptor targeting as an effective immunotherapy against triple-negative breast cancer. BioRxiv, (2020)
V. Piccolo et al., Opposing macrophage polarization programs show extensive epigenomic and transcriptional cross-talk. Nat. Immunol. 18(5), 530–540 (2017)
A. Rodriguez-Garcia et al., CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat. Commun. 12(1), 1–17 (2021)
H.-W. Wang, J.A. Joyce, Alternative activation of tumor-associated macrophages by IL-4: priming for protumoral functions. Cell Cycle 9(24), 4824–4835 (2010)
Y. Lin, J. Xu, H. Lan, Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. Oncol. 12(1), 1–16 (2019)
N. Xue et al., Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci. Rep. 7(1), 1–11 (2017)
X. Yan et al., Lewis lung cancer cells promote SIGNR1 (CD209b)-mediated macrophages polarization induced by IL‐4 to facilitate immune evasion. J. Cell. Biochem. 117(5), 1158–1166 (2016)
S. Setrerrahmane, H. Xu, Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol. Cancer 16(1), 1–17 (2017)
S. Gupta et al., IL-6 augments IL-4-induced polarization of primary human macrophages through synergy of STAT3, STAT6 and BATF transcription factors. Oncoimmunology 7(10), e1494110 (2018)
S. Ito et al., IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model. Cancer Immunol. Immunother. 66(11), 1485–1496 (2017)
G.R. Gunassekaran et al., M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials 278, 121137 (2021)
G.R. Gunassekaran et al., Non-genetic engineering of cytotoxic T cells to target IL-4 receptor enhances tumor homing and therapeutic efficacy against melanoma. Biomaterials 159, 161–173 (2018)
E.B. Nejad et al., IL-6 signaling in macrophages is required for immunotherapy-driven regression of tumors. J. Immunother. Cancer 9, 4 (2021)
H. Liu, J. Shen, K. Lu, IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem. Biophys. Res. Commun. 486(2), 239–244 (2017)
P. Chomarat et al., IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 1(6), 510–514 (2000)
N. Higashino et al., Fibroblast activation protein-positive fibroblasts promote tumor progression through secretion of CCL2 and interleukin-6 in esophageal squamous cell carcinoma. Lab. Invest. 99(6), 777–792 (2019)
Q. Wang et al., Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat. Commun. 9(1), 1–15 (2018)
D.T. Fisher, M.M. Appenheimer, S.S. Evans, The two faces of IL-6 in the tumor microenvironment. Sem. Immunol. 26(1), 38–47 (2014)
M. Papaspyridonos et al., Id1 suppresses anti-tumour immune responses and promotes tumour progression by impairing myeloid cell maturation. Nat. Commun. 6(1), 1–13 (2015)
F. Yang et al., Synergistic immunotherapy of glioblastoma by dual targeting of IL-6 and CD40. Nat. Commun. 12(1), 1–15 (2021)
Y. Wang et al., Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 7(5), 1106–1115 (2014)
X. Ye et al., Oncogenic potential of truncated RXRα during colitis-associated colorectal tumorigenesis by promoting IL-6-STAT3 signaling. Nat. Commun. 10(1), 1–15 (2019)
C. Raggi et al., Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene 35(6), 671–682 (2016)
M. Jiang et al., Interleukin-6 trans-signaling pathway promotes immunosuppressive myeloid-derived suppressor cells via suppression of suppressor of cytokine signaling 3 in breast cancer. Front. Immunol. 8, 1840 (2017)
Y.-C. Wang et al., USP24 induces IL-6 in tumor-associated microenvironment by stabilizing p300 and β-TrCP and promotes cancer malignancy. Nat. Commun. 9(1), 1–18 (2018)
K. Mortezaee et al., NADPH oxidase as a target for modulation of radiation response; implications to carcinogenesis and radiotherapy. Curr. Mol. Pharmacol. 12(1), 50–60 (2019)
J. Mauer, J.L. Denson, J.C. Brüning, Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 36(2), 92–101 (2015)
C. Dominguez et al., Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight 2, 21 (2017)
J. Wen et al., IL-8 promotes cell migration through regulating EMT by activating the Wnt/β‐catenin pathway in ovarian cancer. J. Cell. Mol. Med. 24(2), 1588–1598 (2020)
K.C. Yuen et al., High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 26(5), 693–698 (2020)
K. Kai et al., Oral squamous cell carcinoma contributes to differentiation of monocyte-derived tumor-associated macrophages via PAI-1 and IL-8 production. Int. J. Mol. Sci. 22(17), 9475 (2021)
S. Thongchot et al., Interleukin–8 released by cancer–associated fibroblasts attenuates the autophagy and promotes the migration of ovarian cancer cells. Int. J. Oncol. 58(5), 1–14 (2021)
T. Hasan et al., Interleukin-8/CXCR2 signaling regulates therapy-induced plasticity and enhances tumorigenicity in glioblastoma. Cell Death Dis. 10(4), 1–17 (2019)
T. Zheng et al., IL-8 secreted from M2 macrophages promoted prostate tumorigenesis via STAT3/MALAT1 pathway. Int. J. Mol. Sci. 20(1), 98 (2019)
G. Nie et al., Tumor-associated macrophages-mediated CXCL8 infiltration enhances breast cancer metastasis: Suppression by Danirixin. Int. Immunopharmacol. 95, 107153 (2021)
M. Huang et al., Macrophage inhibitory cytokine-1 induced by a high‐fat diet promotes prostate cancer progression by stimulating tumor‐promoting cytokine production from tumor stromal cells. Cancer Commun. 41(5), 389–403 (2021)
K. Mortezaee. J. Majidpoor, (Im)maturity in tumor ecosystem. Front. Oncol. 11, 813897 (2022)
M. Sanmamed et al., Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. 28(8), 1988–1995 (2017)
Z.A. Lopez-Bujanda et al., Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression. Nat. Cancer 2(8), 803–818 (2021)
R.P. Tobin et al., IL-6 and IL-8 are linked with myeloid-derived suppressor cell accumulation and correlate with poor clinical outcomes in melanoma patients. Front. Oncol. 9, 1223 (2019)
M.H. Mannino et al., The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 367(2), 103–107 (2015)
Y. Chen, W. Tan, C. Wang, Tumor-associated macrophage-derived cytokines enhance cancer stem-like characteristics through epithelial–mesenchymal transition. OncoTargets Therapy 11, 3817 (2018)
W.E. Ip et al., Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356(6337), 513–519 (2017)
K.L. Dennis et al., Current status of IL-10 and regulatory T-cells in cancer. Curr. Opin. Oncol. 25(6), 637 (2013)
E.F. Giunta et al., Baseline IFN-γ and IL-10 expression in PBMCs could predict response to PD-1 checkpoint inhibitors in advanced melanoma patients. Sci. Rep. 10(1), 1–11 (2020)
Y. Zhuang et al., Resistance mechanism of PD-1/PD-L1 blockade in the cancer-immunity cycle. OncoTargets Therapy 13, 83 (2020)
B. Ruffell et al., Macrophage IL-10 blocks CD8 + T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26(5), 623–637 (2014)
J. Qiao et al., Targeting tumors with IL-10 prevents dendritic cell-mediated CD8+ T cell apoptosis. Cancer Cell 35(6): 901–915 (2019)
K. He et al., Cryo-thermal therapy induces macrophage polarization for durable anti-tumor immunity. Cell Death Dis. 10(3), 1–16 (2019)
Y.R. Na et al., The early synthesis of p35 and activation of CDK5 in LPS-stimulated macrophages suppresses interleukin-10 production. Sci. Signal. 8(404), ra121–ra121 (2015)
M. Almanan et al., IL-10–producing Tfh cells accumulate with age and link inflammation with age-related immune suppression. Sci. Adv. 6(31), eabb0806 (2020)
A. Suzuki et al., Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine 75(1), 79–88 (2015)
M. Xiao et al., SENP3 loss promotes M2 macrophage polarization and breast cancer progression. Mol. Oncol. 16(4), 1026–1044 (2022)
X.-X. Deng et al., Taraxacum mongolicum extract inhibited malignant phenotype of triple-negative breast cancer cells in tumor-associated macrophages microenvironment through suppressing IL-10/STAT3/PD-L1 signaling pathways. J. Ethnopharmacol. 274, 113978 (2021)
E. Conte, Targeting monocytes/macrophages in fibrosis and cancer diseases: Therapeutic approaches. Pharmacol. Therap. 11, 108031 (2021)
K. Mortezaee et al., Metformin as a radiation modifier; implications to normal tissue protection and tumor sensitization. Curr. Clin. Pharmacol. 14(1), 41–53 (2019)
K. Mortezaee, Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and liver fibrosis: A review. Cell Biochem. Funct. 36(6), 292–302 (2018)
K. Mortezaee et al., Post-treatment of melatonin with CCl4 better reduces fibrogenic and oxidative changes in liver than melatonin co‐treatment. J. Cell. Biochem. 119(2), 1716–1725 (2018)
G.-Y. Liou et al., The presence of interleukin-13 at pancreatic ADM/PanIN lesions alters macrophage populations and mediates pancreatic tumorigenesis. Cell Rep. 19(7), 1322–1333 (2017)
A.C. Little et al., IL-4/IL-13 stimulated macrophages enhance breast cancer invasion via rho-GTPase regulation of synergistic VEGF/CCL-18 signaling. Front. Oncol. 9, 456 (2019)
C.N. Noto et al., IL-13 acts directly on gastric epithelial cells to promote metaplasia development during chronic gastritis. Cell. Mol. Gastroenterol. Hepatol. 13(2), 623–642 (2022)
J.P. Newman et al., Interleukin-13 receptor alpha 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nat. Commun. 8(1), 1–17 (2017)
R. Bhardwaj et al., Identification of a novel role of IL-13Rα2 in human Glioblastoma multiforme: Interleukin-13 mediates signal transduction through AP-1 pathway. J. Transl. Med. 16(1), 1–13 (2018)
M. Akimoto et al., Interleukin-33 enhances programmed oncosis of ST2L-positive low-metastatic cells in the tumour microenvironment of lung cancer. Cell Death Dis. 7(1), e2057-e2057 (2016)
A. De Boeck et al., Glioma-derived IL-33 orchestrates an inflammatory brain tumor microenvironment that accelerates glioma progression. Nat. Commun. 11(1), 1–24 (2020)
C. Kudo-Saito et al., IL33 is a key driver of treatment resistance of cancer. Cancer Res. 80(10), 1981–1990 (2020)
W. Wang et al., Exogenous interleukin-33 promotes hepatocellular carcinoma growth by remodelling the tumour microenvironment. J. Transl. Med. 18(1), 1–15 (2020)
A. Hatzioannou et al., An intrinsic role of IL-33 in T reg cell–mediated tumor immunoevasion. Nat. Immunol. 21(1), 75–85 (2020)
S. Taniguchi et al., Tumor-initiating cells establish an IL-33–TGF-β niche signaling loop to promote cancer progression. Science 369, 6501 (2020)
J.B. Kamphuis et al., Comment on “Tumor-initiating cells establish an IL-33–TGF-β niche signaling loop to promote cancer progression”. Science 372, 6538 (2021)
J. Wu et al., Interleukin-33 is a novel immunosuppressor that protects cancer cells from TIL killing by a macrophage‐mediated shedding mechanism. Adv. Sci. 8(21), 2101029 (2021)
C. Recio et al., Signal transducer and activator of transcription (STAT)-5: An opportunity for drug development in oncohematology. Oncogene 38(24), 4657–4668 (2019)
Y. Liu et al., IL-2 regulates tumor-reactive CD8 + T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 22(3), 358–369 (2021)
Y. Toyoshima et al., IL6 modulates the immune status of the tumor microenvironment to facilitate metastatic colonization of colorectal cancer cells. Cancer Immunol. Res. 7(12), 1944–1957 (2019)
K. Kashfi, J. Kannikal, N. Nath, Macrophage reprogramming and cancer therapeutics: Role of iNOS-derived NO. Cells 10(11), 3194 (2021)
I.-Y. Kuo et al., Converged Rab37/IL-6 trafficking and STAT3/PD-1 transcription axes elicit an immunosuppressive lung tumor microenvironment. Theranostics 11(14), 7029 (2021)
X. Ju et al., Tumor-associated macrophages induce PD-L1 expression in gastric cancer cells through IL-6 and TNF-ɑ signaling. Exp. Cell Res. 396(2), 112315 (2020)
E. Shklovskaya, H. Rizos, spatial and temporal changes in PD-L1 expression in cancer: The role of genetic drivers, tumor microenvironment and resistance to therapy. Int. J. Mol. Sci. 21(19), 7139 (2020)
J. Majidpoor, K. Mortezaee, The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin. Immunol. 226, 108707 (2021)
S. Arora et al., Comprehensive integrative analysis reveals the association of KLF4 with macrophage infiltration and polarization in lung cancer microenvironment. Cells 10(8), 2091 (2021)
C. Fu et al., Activation of the IL-4/STAT6 signaling pathway promotes lung cancer progression by increasing M2 myeloid cells. Front. Immunol. 10, 2638 (2019)
K. Binnemars-Postma et al., Targeting the Stat6 pathway in tumor‐associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 32(2), 969–978 (2018)
O.M. Rahal et al., Blocking interleukin (IL) 4-and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int. J. Rad. Oncol. Biol. Phys. 100(4), 1034–1043 (2018)
M. Najafi, K. Mortezaee, J. Majidpoor, Stromal reprogramming: a target for tumor therapy. Life Sci. 239, 117049 (2019)
K. Mortezaee, Hypoxia induces core-to-edge transition of progressive tumoral cells: A critical review on differential yet corroborative roles for HIF-1α and HIF-2α. Life Sci. 242, 117145 (2020)
K. Mortezaee, J. Majidpoor, Key promoters of tumor hallmarks. Int. J. Clin. Oncol. 27(1), 45–58 (2022)
J. Majidpoor, K. Mortezaee, Angiogenesis as a hallmark of solid tumors-clinical perspectives. Cell. Oncol. 44(4), 715–737 (2021)
K. Mortezaee, J. Majidpoor, The impact of hypoxia on immune state in cancer. Life Sci. 286, 120057 (2021)
K. Mortezaee et al., Targets for improving tumor response to radiotherapy. Int. Immunopharmacol. 76, 105847 (2019)
M.T. Munir et al., Tumor-associated macrophages as multifaceted regulators of breast tumor growth. Int. J. Mol. Sci. 22(12), 6526 (2021)
W. Jin, Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial–mesenchymal transition. Cells 9(1), 217 (2020)
R. Vazquez-Lombardi et al., Potent antitumour activity of interleukin-2-Fc fusion proteins requires Fc-mediated depletion of regulatory T-cells. Nat. Commun. 8(1), 1–12 (2017)
N. Arenas-Ramirez et al., Improved cancer immunotherapy by a CD25-mimobody conferring selectivity to human interleukin-2. Sci. Transl. Med. 8(367), 367ra166–367ra166 (2016)
E.J. Hsu et al., A cytokine receptor-masked IL2 prodrug selectively activates tumor-infiltrating lymphocytes for potent antitumor therapy. Nat. Commun. 12(1), 1–13 (2021)
M. Sharma et al., Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy. Nat. Commun. 11(1), 1–11 (2020)
L.B. Schenkel et al., A potent and selective PARP14 inhibitor decreases protumor macrophage gene expression and elicits inflammatory responses in tumor explants. Cell Chem. Biol. 28(8), 1158–1168 (2021)
G.R. Gunassekaran et al., M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials 278, 121137 (2021)
C. Yang et al., CD44 v5 domain inhibition represses the polarization of Th2 cells by interfering with the IL-4/IL‐4R signaling pathway. Immunol. Cell Biol. 100(1), 21–32 (2022)
H. Harb, T.A. Chatila, Mechanisms of dupilumab. Clin. Exp. Allergy 50(1), 5–14 (2020)
A. Le Floc’h et al., Dual blockade of IL-4 and IL‐13 with dupilumab, an IL‐4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 75(5), 1188–1204 (2020)
E. Guttman-Yassky et al., Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatology 156(4), 411–420 (2020)
M.C. Matsunaga, P.S. Yamauchi, IL-4 and IL-13 inhibition in atopic dermatitis. J. Drugs Dermatol. 15(8), 925–929 (2016)
C. Hahn et al., Inhibition of the IL-4/IL-13 receptor system prevents allergic sensitization without affecting established allergy in a mouse model for allergic asthma. J. Allergy Clin. Immunol. 111(6), 1361–1369 (2003)
J. Majidpoor, K. Mortezaee, Interleukin-6 in SARS-CoV-2 induced disease: Interactions and therapeutic applications. Biomed. Pharmacother. 145, 112419 (2022)
E. Dijkgraaf et al., A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti-IL-6R monoclonal antibody, and interferon-α2b in patients with recurrent epithelial ovarian cancer. Ann. Oncol. 26(10), 2141–2149 (2015)
H. Zhong et al., A novel IL6 antibody sensitizes multiple tumor types to chemotherapy including trastuzumab-resistant tumors. Cancer Res. 76(2), 480–490 (2016)
K.A. Finkel et al., IL-6 inhibition with MEDI5117 decreases the fraction of head and neck cancer stem cells and prevents tumor recurrence. Neoplasia 18(5), 273–281 (2016)
M. Bilusic et al., Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 7(1), 1–8 (2019)
J.W. Greiner, Y.M. Morillon, J.S. II, NHS-IL12, a Tumor-Targeting Immunocytokine. ImmunoTargets Therapy 10, 155 (2021)
Y. Sun et al., Co-delivery of IL-12 cytokine gene and cisplatin prodrug by a polymetformin-conjugated nanosystem for lung cancer chemo-gene treatment through chemotherapy sensitization and tumor microenvironment modulation. Acta Biomater. 128(1), 447–461 (2021)
S.K. Watkins et al., IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J. Immunol. 178(3), 1357–1362 (2007)
T. Satoh et al., Macrophages transduced with an adenoviral vector expressing interleukin 12 suppress tumor growth and metastasis in a preclinical metastatic prostate cancer model. Cancer Res. 63(22), 7853–7860 (2003)
N. Qiu et al., Tumor-associated macrophage and tumor‐cell dually transfecting polyplexes for efficient interleukin‐12 cancer gene therapy. Adv. Mater. 33(2), 2006189 (2021)
K.J. Brempelis et al., Genetically engineered macrophages persist in solid tumors and locally deliver therapeutic proteins to activate immune responses. J. Immunother. Cancer 8(2), e001356 (2020)
J.A. Thompson et al., Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J. Clin. Oncol. 26(12), 2034–2039 (2008)
S. Bhatia et al., Recombinant interleukin-21 plus sorafenib for metastatic renal cell carcinoma: a phase 1/2 study. J. Immunother. Cancer 2(1), 1–11 (2014)
S. Wu et al., The half-life-extended IL21 can be combined with multiple checkpoint inhibitors for tumor immunotherapy. Front. Cell Dev. Biol. 9, 779865 (2021)
V. Cervera-Carrascon et al., Adenovirus armed with TNFa and IL2 added to aPD-1 regimen mediates antitumor efficacy in tumors refractory to aPD-1. Front. Immunol. 12, 706517 (2021)
N.H. Goradel et al., Oncolytic adenovirus: A tool for cancer therapy in combination with other therapeutic approaches. J. Cell. Physiol. 234(6), 8636–8646 (2019)
W. Liu et al., In Situ therapeutic cancer vaccination with an oncolytic virus expressing Membrane-Tethered IL-2. Mol. Therapy-Oncolytics 17, 350–360 (2020)
Y.R. Suryawanashi et al., T-independent response mediated by oncolytic tanapoxvirus recombinants expressing interleukin-2 and monocyte chemoattractant protein-1 suppresses human triple negative breast tumors. Med. Oncol. 34(6), 112 (2017)
T. Chen et al., IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. J. Immunother. Cancer 9(1), e001647 (2021)
Y. Jia et al., Associations of common IL-4 gene polymorphisms with cancer risk: A meta-analysis. Mol. Med. Rep. 16(2), 1927–1945 (2017)
V. Subbiah et al., Cytokines produced by dendritic cells administered intratumorally correlate with clinical outcome in patients with diverse cancers. Clin. Cancer Res. 24(16), 3845–3856 (2018)
A. Naing et al., PEGylated IL-10 (Pegilodecakin) induces systemic immune activation, CD8 + T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell 34(5): 775–791 (2018)
A. Naing et al., Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol. 20(11), 1544–1555 (2019)
C. Stolfi et al., Interleukin-21 in cancer immunotherapy: Friend or foe? Oncoimmunology 1(3), 351–354 (2012)
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
K.M initiated the conceptualization. J.M and K.M wrote the initial manuscript. Final revisions were made by K.M. Articles were selected by K.M. Both authors approved the final draft.
Corresponding author
Ethics declarations
Ethical Approval and Consent to participate
This work has received the ethical code IR.MUK.REC.1400.208 from Kurdistan University of Medical Sciences. Consent to participate is not applicable.
Human Ethics and Animal Ethics statements
Not applicable.
Competing interests/Conflicts of interest
The authors declare that they have no conflict of interest.
Availability of supporting data
Data sharing is not applicable to this article, as no datasets were generated or analysed during the current study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mortezaee, K., Majidpoor, J. Roles for macrophage-polarizing interleukins in cancer immunity and immunotherapy. Cell Oncol. 45, 333–353 (2022). https://doi.org/10.1007/s13402-022-00667-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-022-00667-8