Abstract
As one of the main brewing by-products, Saccharomyces cerevisiae extracts (from spent yeast) have been commercialized as food supplement for years. Among their several claims, the application as protein source is highlighted. In fact, their high protein content (about 45–60%) including essential amino acids with high biological value, safety and low cost are primarily responsible for their spreading in agri-food sector. Meanwhile, cosmetic and health sectors have been working on yeast bioactive peptides because of their antihypertensive, antioxidant and antimicrobial properties, among others. Several studies related to valorisation of S. cerevisiae are currently ongoing, aiming to create novel products and optimize production processes. The present review aims to provide an overview from production of protein-rich extracts from S. cerevisiae to their chemical characterisation, detailing protein extraction, isolation and purification processes, as well as characterisation methods for the final extracts.
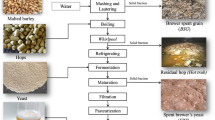
Graphical abstract




Similar content being viewed by others
References
Hayes M (2018) Food proteins and bioactive peptides: new and novel sources, characterisation strategies and applications. Foods 7:38. https://doi.org/10.3390/foods7030038
Food and Agriculture Organization of the United Nations (2018) The future of food and agriculture – alternative pathways to 2050. http://www.fao.org/global-perspectives-studies/resources/detail/en/c/1157082/. Accessed 26 Nov 2021
Henchion M, Hayes M, Mullen A et al (2017) Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Foods 6:53. https://doi.org/10.3390/foods6070053
Okolie CL, Akanbi TO, Mason B et al (2019) Influence of conventional and recent extraction technologies on physicochemical properties of bioactive macromolecules from natural sources: a review. Food Res Int 116:827–839. https://doi.org/10.1016/j.foodres.2018.09.018
Transparency Market Research (2020) Peptide therapeutics market- global industry analysis, size, share, growth, trends, and forecast 2019 - 2027. https://www.transparencymarketresearch.com/peptide-therapeutics-market.html. Accessed 26 Nov 2021
Mirzaei M, Mirdamadi S, Safavi M (2019) Antioxidant activity and protective effects of Saccharomyces cerevisiae peptide fractions against H 2 O 2 - induced oxidative stress in Caco - 2 cells. J Food Meas Charact 13:2654–2662. https://doi.org/10.1007/s11694-019-00186-5
Gddoa Al-sahlany ST, Altemimi AB, Abd Al-Manhel AJ et al (2020) Purification of bioactive peptide with antimicrobial properties produced by Saccharomyces cerevisiae. Foods 9:1–11. https://doi.org/10.3390/foods9030324
Jung EY, Lee HS, Choi JW et al (2011) Glucose tolerance and antioxidant activity of spent brewer’s yeast hydrolysate with a high content of cyclo-his-pro (CHP). J Food Sci 76:272–278. https://doi.org/10.1111/j.1750-3841.2010.01997.x
Kim KM, Chang UJ, Kang DH et al (2004) Yeast hydrolysate reduces body fat of dietary obese rats. Phytother Res 18:950–953. https://doi.org/10.1002/ptr.1582
Indumathi P, Mehta A (2016) A novel anticoagulant peptide from the Nori hydrolysate. J Funct Foods 20:606–617. https://doi.org/10.1016/j.jff.2015.11.016
Amorim M, Marques C, Pereira JO et al (2019) Antihypertensive effect of spent brewer yeast peptide. Process Biochem 76:213–218. https://doi.org/10.1016/j.procbio.2018.10.004
de la Hoz L, Ponezi AN, Milani RF et al (2014) Iron-binding properties of sugar cane yeast peptides. Food Chem 142:166–169. https://doi.org/10.1016/j.foodchem.2013.06.133
Marson GV, de Castro RJS, Belleville MP, Hubinger MD (2020) Spent brewer’s yeast as a source of high added value molecules: a systematic review on its characteristics, processing and potential applications. World J Microbiol Biotechnol 36:1–22. https://doi.org/10.1007/s11274-020-02866-7
Food Agricultural Organization of the United Nations (2019) Food balance sheets: protein supply quantity (g/capita/day). http://www.fao.org/faostat/en/#data/FBS. Accessed 26 Jan 2022
Sá AGA, Moreno YMF, Carciofi BAM (2020) Plant proteins as high-quality nutritional source for human diet. Trends Food Sci Technol 97:170–184. https://doi.org/10.1016/j.tifs.2020.01.011
Hayes M, Mora L, Hussey K, Aluko RE (2016) Boarfish protein recovery using the pH-shift process and generation of protein hydrolysates with ACE-I and antihypertensive bioactivities in spontaneously hypertensive rats. Innov Food Sci Emerg Technol 37:253–260. https://doi.org/10.1016/j.ifset.2016.03.014
Akhtar Y, Isman MB (2018) Insects as an alternative protein source. In: Proteins in food processing. Elsevier, pp 263–288. https://doi.org/10.1016/B978-0-08-100722-8.00011-5
Ritala A, Häkkinen ST, Toivari M, Wiebe MG (2017) Single cell protein-state-of-the-art, industrial landscape and patents 2001–2016. Front Microbiol. https://doi.org/10.3389/fmicb.2017.02009
Nasseri AT, Rasoul-Ami S, Morowvat MH, Ghasemi Y (2011) Single cell protein: production and process. Am J Food Technol 6:103–116. https://doi.org/10.3923/ajft.2011.103.116
Jones SW, Karpol A, Friedman S et al (2020) Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr Opin Biotechnol 61:189–197. https://doi.org/10.1016/j.copbio.2019.12.026
Tibbetts SM (2018) The potential for ‘next-generation’, microalgae-based feed ingredients for salmonid aquaculture in context of the blue revolution. In: Microalgal biotechnology. InTech
World Health Organization (2007) Protein and amino acid requirements in human nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation
Kuhad RC, Singh A, Tripathi KK et al (1997) Microorganisms as an alternative source of protein. Nutr Rev 55:65–75
Rudravaram R, Chandel AK, Rao LV, et al (2009) Bio (single cell) protein: issues of production, toxins and commercialisation status. In: Agricultural wastes. pp 129–153
Fleet GH (2007) Yeasts in foods and beverages: impact on product quality and safety. Curr Opin Biotechnol 18:170–175. https://doi.org/10.1016/j.copbio.2007.01.010
Pereira PR, Freitas CS, Paschoalin VMF (2021) Saccharomyces cerevisiae biomass as a source of next-generation food preservatives: evaluating potential proteins as a source of antimicrobial peptides. Compr Rev Food Sci Food Saf 20:4450–4479. https://doi.org/10.1111/1541-4337.12798
Fărcaş AC, Socaci SA, Mudura E et al (2017) Exploitation of brewing industry wastes to produce functional ingredients. Brew Technol. https://doi.org/10.5772/intechopen.69231
García-Garibay M, Gómez-Ruiz L, Cruz-Guerrero AE, Bárzana E (2014) Single cell protein: yeasts and bacteria. In: Encyclopedia of food microbiology. Elsevier, pp 431–438
Kurcz A, Błażejak S, Kot AM et al (2018) Application of industrial wastes for the production of microbial single-cell protein by fodder yeast Candida utilis. Waste and Biomass Valorization 9:57–64. https://doi.org/10.1007/s12649-016-9782-z
Bombe K (2019) Specialty yeast market by type (yeast extract, yeast autolysate, yeast beta - glucan), application (bakery production, flavoring, biofuels), species (Saccharomyces Cerevisiae, Kluyveromyces), and industry – global forecast to 2025. https://www.meticulousresearch.com/product/specialty-yeast-market-5032/?utm_source=Globnewswire.com&utm_medium=PressRelease&utm_campaign=Paid
Jaeger A, Arendt EK, Zannini E (2020) Brewer ’ s spent yeast ( BSY ), an underutilized brewing by-product. 1–23. https://doi.org/10.3390/fermentation6040123
Rakowska R, Sadowska A, Dybkowska E, Świderski F (2017) Spent yeast as natural source of functional food additives. Rocz Panstw Zakl Hig 68:115–121
Payen C, Thompson D (2019) The renaissance of yeasts as microbial factories in the modern age of biomanufacturing. Yeast 36:685–700. https://doi.org/10.1002/yea.3439
Stewart GG (2016) Saccharomyces species in the production of beer. Beverages. https://doi.org/10.3390/beverages2040034
Conway J (2021) Beer production worldwide from 1998 to 2020. https://www.statista.com/statistics/270275/worldwide-beer-production/. Accessed 26 Nov 2021
Cooray ST, Lee JJL, Chen WN (2017) Evaluation of brewers’ spent grain as a novel media for yeast growth. AMB Express 7:117. https://doi.org/10.1186/s13568-017-0414-1
Vieira EF, Cunha SC, Ferreira IMPLVO (2019) Characterization of a potential bioactive food ingredient from inner cellular content of brewer’s spent yeast. Waste and Biomass Valorization 10:3235–3242. https://doi.org/10.1007/s12649-018-0368-9
Feldmann H (2012) Yeast cell architecture and functions. Yeast: molecular and cell biology. Second Edi. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, pp 5–24
Klis FM, Mol P, Hellingwerf K, Brul S (2002) Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol Rev 26:239–256. https://doi.org/10.1111/j.1574-6976.2002.tb00613.x
Faustino M, Durão J, Pereira CF et al (2021) Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae – a sustainable source of functional ingredients. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2021.118467
Wang J, Li M, Zheng F et al (2018) Cell wall polysaccharides: before and after autolysis of brewer’s yeast. World J Microbiol Biotechnol 34:137. https://doi.org/10.1007/s11274-018-2508-6
Orlean P (2012) Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 192:775–818. https://doi.org/10.1534/genetics.112.144485
Liu D, Ding L, Sun J et al (2016) Yeast cell disruption strategies for recovery of intracellular bio-active compounds — a review. Innov Food Sci Emerg Technol 36:181–192. https://doi.org/10.1016/j.ifset.2016.06.017
Jacob FF, Hutzler M, Methner F-J (2019) Comparison of various industrially applicable disruption methods to produce yeast extract using spent yeast from top-fermenting beer production: influence on amino acid and protein content. Eur Food Res Technol 245:95–109. https://doi.org/10.1007/s00217-018-3143-z
Jamshad M, Darby RAJ (2012) Disruption of yeast cells to isolate recombinant proteins. In: Methods in molecular biology. pp 237–246
Bzducha-Wróbel A, Błażejak S, Kawarska A et al (2014) Evaluation of the efficiency of different disruption methods on yeast cell wall preparation for β-glucan isolation. Molecules 19:20941–20961. https://doi.org/10.3390/molecules191220941
del Contreras M, M, Lama-Muñoz A, Manuel Gutiérrez-Pérez J, et al (2019) Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour Technol 280:459–477. https://doi.org/10.1016/j.biortech.2019.02.040
Ekpeni LEN, Benyounis KY, Nkem-Ekpeni FF et al (2015) Underlying factors to consider in improving energy yield from biomass source through yeast use on high-pressure homogenizer (hph). Energy 81:74–83. https://doi.org/10.1016/j.energy.2014.11.038
Gaver D, Huyghebaert A (1991) Optimization of yeast cell disruption with a newly designed bead mill. Enzyme Microb Technol 13:665–671. https://doi.org/10.1016/0141-0229(91)90082-L
Middelberg APJ (1995) Process-scale disruption of microorganisms. Biotechnol Adv 13:491–551. https://doi.org/10.1016/0734-9750(95)02007-P
Currie JA, Dunnill P, Lilly MD (1972) Release of protein from Bakers’ yeast (Saccharomyces cerevisiae) by disruption in an industrial agitator mill. Biotechnol Bioeng 14:725–736. https://doi.org/10.1002/bit.260140504
Jacob FF, Striegel L, Rychlik M et al (2019) Yeast extract production using spent yeast from beer manufacture: influence of industrially applicable disruption methods on selected substance groups with biotechnological relevance. Eur Food Res Technol 245:1169–1182. https://doi.org/10.1007/s00217-019-03237-9
Hedenskog G, Mogren H (1973) Some methods for processing of single-cell protein. Biotechnol Bioeng 15:129–142. https://doi.org/10.1002/bit.260150110
Koubaa M, Imatoukene N, Drévillon L, Vorobiev E (2020) Current insights in yeast cell disruption technologies for oil recovery: a review. Chem Eng Process - Process Intensif 150:107868. https://doi.org/10.1016/j.cep.2020.107868
Bystryak S, Santockyte R, Peshkovsky AS (2015) Cell disruption of S. cerevisiae by scalable high-intensity ultrasound. Biochem Eng J 99:99–106. https://doi.org/10.1016/j.bej.2015.03.014
Ekpeni LEN, Benyounis KY, Stokes J, Olabi AG (2016) Improving and optimizing protein concentration yield from homogenized Baker’s yeast at different ratios of buffer solution. Int J Hydrogen Energy 41:16415–16427. https://doi.org/10.1016/j.ijhydene.2016.05.243
Balasundaram B, Harrison STL (2008) Influence of the extent of disruption of Bakers’ yeast on protein adsorption in expanded beds. J Biotechnol 133:360–369. https://doi.org/10.1016/j.jbiotec.2007.07.724
Siddiqi SF, Titchener-Hooker NJ, Shamlou PA (1997) High pressure disruption of yeast cells: The use of scale down operations for the prediction of protein release and cell debris size distribution. Biotechnol Bioeng 55:642–649. https://doi.org/10.1002/(SICI)1097-0290(19970820)55:4%3c642::AID-BIT6%3e3.0.CO;2-H
Liu D, Lebovka NI, Vorobiev E (2013) Impact of electric pulse treatment on selective extraction of intracellular compounds from Saccharomyces cerevisiae yeasts. Food Bioprocess Technol 6:576–584. https://doi.org/10.1007/s11947-011-0703-7
Lin HM, Chan EC, Chen C, Chen LF (1991) Disintegration of yeast cells by pressurized carbon dioxide. Biotechnol Prog 7:201–204. https://doi.org/10.1021/bp00009a001
Lin HM, Yang Z, Chen LF (1992) An improved method for disruption of microbial cells with pressurized carbon dioxide. Biotechnol Prog 8:165–166. https://doi.org/10.1021/bp00014a012
Kadam SU, Tiwari BK, Álvarez C, O’Donnell CP (2015) Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci Technol 46:60–67. https://doi.org/10.1016/j.tifs.2015.07.012
Zhang L, Jin Y, Xie Y et al (2014) Releasing polysaccharide and protein from yeast cells by ultrasound: selectivity and effects of processing parameters. Ultrason Sonochem 21:576–581. https://doi.org/10.1016/j.ultsonch.2013.10.016
Wu T, Yu X, Hu A et al (2015) Ultrasonic disruption of yeast cells: underlying mechanism and effects of processing parameters. Innov Food Sci Emerg Technol 28:59–65. https://doi.org/10.1016/j.ifset.2015.01.005
James CJ, Coakley WT, Hughes DE (1972) Kinetics of protein release from yeast sonicated in batch and flow systems at 20 kHz. Biotechnol Bioeng 14:33–42. https://doi.org/10.1002/bit.260140105
Apar DK, Özbek B (2008) Protein releasing kinetics of bakers’ yeast cells by ultrasound. Chem Biochem Eng Q 22:113–118
Iida Y, Tuziuti T, Yasui K et al (2008) Protein release from yeast cells as an evaluation method of physical effects in ultrasonic field. Ultrason Sonochem 15:995–1000. https://doi.org/10.1016/j.ultsonch.2008.02.013
Liu D, Zeng X-AA, Sun D-WW, Han Z (2013) Disruption and protein release by ultrasonication of yeast cells. Innov Food Sci Emerg Technol 18:132–137. https://doi.org/10.1016/j.ifset.2013.02.006
Agrawal PB, Pandit AB (2003) Isolation of α-glucosidase from Saccharomyces cerevisiae: cell disruption and adsorption. Biochem Eng J 15:37–45. https://doi.org/10.1016/S1369-703X(02)00178-X
Ganeva V, Galutzov B, Teissié J (2003) High yield electroextraction of proteins from yeast by a flow process. Anal Biochem 315:77–84. https://doi.org/10.1016/S0003-2697(02)00699-1
Ganeva V, Galutzov B (1999) Electropulsation as an alternative method for protein extraction from yeast. FEMS Microbiol Lett 174:279–284. https://doi.org/10.1111/j.1574-6968.1999.tb13580.x
Ohshima T, Sato M, Saito M (1995) Selective release of intracellular protein using pulsed electric field. J Electrostat 35:103–112. https://doi.org/10.1016/0304-3886(95)00014-2
Kim SK (2016) Marine glycobiology: principles and applications, First ed. CRC Press
Klimek-Ochab M, Brzezińska-Rodak M, Zymańczyk-Duda E et al (2011) Comparative study of fungal cell disruption-scope and limitations of the methods. Folia Microbiol (Praha) 56:469–475. https://doi.org/10.1007/s12223-011-0069-2
Kushnirov VV (2000) Rapid and reliable protein extraction from yeast. Yeast 16:857–860. https://doi.org/10.1002/1097-0061(20000630)16:9%3c857::AID-YEA561%3e3.0.CO;2-B
Zhang T, Lei J, Yang H et al (2011) An improved method for whole protein extraction from yeast Saccharomyces cerevisiae. Yeast 28:795–798. https://doi.org/10.1002/yea.1905
Mukherjee M, Nandi A, Chandra K et al (2020) Protein extraction from Saccharomyces cerevisiae at different growth phases. J Microbiol Methods 172:105906. https://doi.org/10.1016/j.mimet.2020.105906
Ge L, Wang XT, Tan SN et al (2010) A novel method of protein extraction from yeast using ionic liquid solution. Talanta 81:1861–1864. https://doi.org/10.1016/j.talanta.2010.02.034
Takalloo Z, Nikkhah M, Nemati R et al (2020) Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): a comparative study. World J Microbiol Biotechnol 36:1–14. https://doi.org/10.1007/s11274-020-02840-3
Podpora B, Swiderski F (2015) Spent brewer’s yeast autolysates as a new and valuable component of functional food and dietary supplements. J Food Process Technol. https://doi.org/10.4172/2157-7110.1000526
Xie J, Cui C, Ren J et al (2017) High solid concentrations facilitate enzymatic hydrolysis of yeast cells. Food Bioprod Process 103:114–121. https://doi.org/10.1016/j.fbp.2017.03.004
Chae HJ, Joo H, In M (2001) Utilization of brewer’s yeast cells for the production of food-grade yeast extract. Part 1: effects of different enzymatic treatments on solid and protein recovery and flavor characteristics. Bioresour Technol 76:253–258. https://doi.org/10.1016/S0960-8524(00)00102-4
Celus I, Brijs K, Delcour JA (2007) Enzymatic hydrolysis of brewers’ spent grain proteins and technofunctional properties of the resulting hydrolysates. J Agric Food Chem 55:8703–8710. https://doi.org/10.1021/jf071793c
Podpora B, Swiderski F, Sadowska A, et al (2016) Spent brewer’s yeast extracts as a new component of functional food. Czech J Food Sci 34:554–563. https://doi.org/10.17221/419/2015-CJFS
Marson GV, Lacour S, Hubinger MD, Belleville MP (2022) Serial fractionation of spent brewer’s yeast protein hydrolysate by ultrafiltration: a peptide-rich product with low RNA content. J Food Eng 312:110737. https://doi.org/10.1016/j.jfoodeng.2021.110737
Hobson J (1991) A co-hydrolytic process for the production of novel extracts from yeast and non-yeast proteins
Kortes J (2020) Process flavours with low acrylamide
Jolly R (1978) Modified protein
Ason K (2019) Effective use of yeast and yeast extract residue
Farra CD (2015) Cosmetic and/or pharmaceutical composition comprising a yeast peptide hydrolysate and use of the yeast peptide hydrolysate as an active agent for strengthening hair
Hedhammar M, Karlström AE, Hober S (2006) Chromatographic methods for protein purification, Royal Institute of Technology, Stockholm, Sweden. Stockholm: Royal Institute of Technology
Liu D, Savoire R, Vorobiev E, Lanoisellé JL (2010) Effect of disruption methods on the dead-end microfiltration behavior of yeast suspension. Sep Sci Technol 45:1042–1050. https://doi.org/10.1080/01496391003727890
Butylina S, Shataeva LK, Nyström M (2007) Separation of nucleoprotein complexes with antioxidant activity from yeast Saccharomyces cerevisiae. Sep Purif Technol 53:64–70. https://doi.org/10.1016/j.seppur.2006.06.014
Caballero-Córdoba GM, Sgarbieri VC (2000) Nutritional and toxicological evaluation of yeast (Saccharomyces cerevisiae) biomass and a yeast protein concentrate. J Sci Food Agric 80:341–351. https://doi.org/10.1002/1097-0010(200002)80:3%3c341::AID-JSFA533%3e3.3.CO;2-D
Yamada EA, Sgarbieri VC (2005) Yeast (Saccharomyces cerevisiae) protein concentrate: preparation, chemical composition, and nutritional and functional properties. J Agric Food Chem 53:3931–3936. https://doi.org/10.1021/jf0400821
Akardere E, Özer B, Çelem EB, Önal S (2010) Three-phase partitioning of invertase from Baker’s yeast. Sep Purif Technol 72:335–339. https://doi.org/10.1016/j.seppur.2010.02.025
Mohammad AW, Ng CY, Lim YP, Ng GH (2012) Ultrafiltration in food processing industry: review on application, membrane fouling, and fouling control. Food Bioprocess Technol 5:1143–1156. https://doi.org/10.1007/s11947-012-0806-9
Vollet Marson G, Belleville M, Lacour S, Dupas Hubinger M (2020) Membrane fractionation of protein hydrolysates from by-products: recovery of valuable compounds from spent yeasts. Membranes (Basel) 11:23. https://doi.org/10.3390/membranes11010023
Kim J, Dae-Hyoung L, Jong-Soo L et al (2004) Characterization of antihypertensive angiotensin I-converting enzyme inhibitor from Saccharomyces cerevisiae. J Microbiol Biotechnol 14:1318–1323
Albergaria H, Francisco D, Gori K et al (2010) Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl Microbiol Biotechnol 86:965–972. https://doi.org/10.1007/s00253-009-2409-6
Branco P, Francisco D, Chambon C et al (2014) Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl Microbiol Biotechnol 98:843–853. https://doi.org/10.1007/s00253-013-5411-y
Mirzaei M, Mirdamadi S, Ehsani MR et al (2015) Purification and identification of antioxidant and ACE-inhibitory peptide from Saccharomyces cerevisiae protein hydrolysate. J Funct Foods 19:259–268
Lee DH, Lee DH, Lee JS (2007) Characterization of a new antidementia β-secretase inhibitory peptide from Saccharomyces cerevisiae. Enzyme Microb Technol 42:83–88. https://doi.org/10.1016/j.enzmictec.2007.08.003
Nehete J, Narkhede M, Bhambar R et al (2013) Natural proteins: sources, isolation, characterization and applications. Pharmacogn Rev 7:107. https://doi.org/10.4103/0973-7847.120508
Clark EDB (2001) Protein refolding for industrial processes. Curr Opin Biotechnol 12:202–207. https://doi.org/10.1016/S0958-1669(00)00200-7
Sui H, Zhou J, Ma G et al (2018) Removal of ionic liquids from oil sands processing solution by ion-exchange resin. Appl Sci 8:1611. https://doi.org/10.3390/app8091611
Dick K, Molan P, Eschenbruch R (1992) The isolation from Saccharomyces cerevisiae of two antibacterial cationic proteins that inhibit malolactic bacteria. Vitis 31:105–116
Grönberg A (2018) Ion exchange chromatography. In: Biopharmaceutical processing: development, design, and implementation of manufacturing processes. Elsevier, pp 379–399
Lothe RR, Purohit SS, Shaikh SS, et al (1999) Purification of α-glucosidae and invertase from Bakers ’ yeast on modified polymeric supports. 293–306. https://doi.org/10.1023/A:1008126628635
Josic D, Kovac S (2010) Reversed-phase high performance liquid chromatography of proteins. Curr Protoc Protein Sci 2010:1–22. https://doi.org/10.1002/0471140864.ps0807s61
Shetty JK, Kinsella JE (1980) Lysinoalanine formation in yeast proteins isolated by alkaline methods. J Agric Food Chem 28:798–800. https://doi.org/10.1021/jf60230a019
Shetty JK, Kinsella JE (1980) Ready separation of proteins from nucleoprotein complexes by reversible modification of lysine residues. Biochem J 191:269–272. https://doi.org/10.1042/bj1910269
Lindblom M (1977) Properties of intracellular ribonuclease utilized for RNA reduction in disintegrated cells of Saccharomyces cerevisiae. Biotechnol Bioeng 19:199–210. https://doi.org/10.1002/bit.260190204
Shetty KJ, Kinsella JE (1979) Preparation of yeast protein isolate with low nucleic acid by succinylation. J Food Sci 44:633–638. https://doi.org/10.1111/j.1365-2621.1979.tb08464.x
Kinsella JE, Damodaran S (1984) Dissociation of yeast nucleoprotein complexes by chemical phosphorylation. J Agric Food Chem 32:1030–1032. https://doi.org/10.1021/jf00125a021
Huang Y-T, Kinsella JE (1986) Phosphorylation of yeast protein: reduction of ribonucleic acid and isolation of yeast protein concentrate. Biotechnol Bioeng 28:1690–1698. https://doi.org/10.1002/bit.260281112
Oliveira AM, de Oliva Neto P (2011) Improvement in RNA extraction from S. cerevisie by optimization in the autolysis and NH3 hydrolysis. Brazilian Arch Biol Technol 54:1007–1018. https://doi.org/10.1590/S1516-89132011000500019
Sombutyanuchit P, Suphantharika M, Verduyn C (2001) Preparation of 5′-GMP-rich yeast extracts from spent brewer’s yeast. World J Microbiol Biotechnol 17:163–168. https://doi.org/10.1023/A:1016686504154
Goetz H, Kuschel M, Wulff T et al (2004) Comparison of selected analytical techniques for protein sizing, quantitation and molecular weight determination. J Biochem Biophys Methods 60:281–293. https://doi.org/10.1016/j.jbbm.2004.01.007
Mæhre HK, Dalheim L, Edvinsen GK et al (2018) Protein determination-method matters. Foods. https://doi.org/10.3390/foods7010005
AOAC (2005) Official methods of analysis of AOAC International
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem. https://doi.org/10.1016/0922-338X(96)89160-4
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. https://doi.org/10.1016/0003-2697(85)90442-7
Puligundla P, Mok C, Park S (2020) Advances in the valorization of spent brewer’s yeast. Innov Food Sci Emerg Technol 62:102350. https://doi.org/10.1016/j.ifset.2020.102350
Rai AK, Pandey A, Sahoo D (2019) Biotechnological potential of yeasts in functional food industry. Trends Food Sci Technol 83:129–137. https://doi.org/10.1016/j.tifs.2018.11.016
Kaltashov IA, Bobst CE, Pawlowski J, Wang G (2020) Mass spectrometry-based methods in characterization of the higher order structure of protein therapeutics. J Pharm Biomed Anal 184:113169. https://doi.org/10.1016/j.jpba.2020.113169
Ryan DJ, Spraggins JM, Caprioli RM (2019) Protein identification strategies in MALDI imaging mass spectrometry: a brief review. Curr Opin Chem Biol 48:64–72. https://doi.org/10.1016/j.cbpa.2018.10.023
Shynkaryk MV, Lebovka NI, Lanoisellé JL et al (2009) Electrically-assisted extraction of bio-products using high pressure disruption of yeast cells (Saccharomyces cerevisiae). J Food Eng 92:189–195. https://doi.org/10.1016/j.jfoodeng.2008.10.041
Funding
This work was co-financed by European Regional Development Fund (ERDF), through the Operational Program for Competitiveness and Internationalization (POCI) under Alchemy project—Capturing high value from industrial fermentation bio products (POCI-01–0247-FEDER-027578).
Author information
Authors and Affiliations
Contributions
A.S. Oliveira drafted the work, being in charge of conceptualisation, literature search and writing; C. Ferreira conduced the conceptualisation, revision and edition; J.O. Pereira contributed for revision and edition; M. E. Pintado leaded the supervision and project administration; A. P. Carvalho was responsible for final revision and edition, and supervision.
Corresponding authors
Ethics declarations
Competing of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oliveira, A.S., Ferreira, C., Pereira, J.O. et al. Valorisation of protein-rich extracts from spent brewer’s yeast (Saccharomyces cerevisiae): an overview. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-02636-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-02636-5