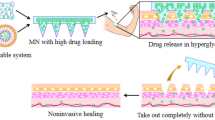
Abstract
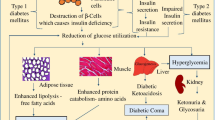
Diabetes is one of the most serious chronic diseases today. Patients with diabetes need frequent insulin injections or blood sampling to monitor blood glucose levels. The microneedles are a painless transdermal drug delivery system, which has great advantages in achieving self-management. There have been a lot of researches on microneedles used in diabetes treatment. Microneedle-based treatment of diabetes has also changed from a simple and reliable system to a complex and efficient system. This review introduces microfluidic, glucose response, and other contents based on microneedles, and some challenges in the development of microneedles.
Graphical abstract




Similar content being viewed by others
References
International Diabetes Federation. IDF Diabetes Atlas Ninth Edition. 2019. Achieved from https://diabetesatlas.org/en/resources/.
Han S-K, Lee S-J, Ha H-Y. Skin moisturizing effects of a microneedle patch containing hyaluronic acid and lonicerae flos. Processes. 2021;9(2):321.
Jang M, Baek S, Kang G, Yang H, Kim S, Jung H. Dissolving microneedle with high molecular weight hyaluronic acid to improve skin wrinkles, dermal density and elasticity. Int J Cosmet Sci. 2020;42(3):302–9.
Kim MC, Kim KH, Lee JW, Lee YN, Choi HJ, Jung YJ, Kim YJ, Compans RW, Prausnitz MR, Kang SM. Co-delivery of M2e virus-like particles with influenza split vaccine to the skin using microneedles enhances the efficacy of cross protection. Pharmaceutics. 2019;11(4):188.
Chen YH, Lai KY, Chiu YH, Wu YW, Shiau AL, Chen MC. Implantable microneedles with an immune-boosting function for effective intradermal influenza vaccination. Acta Biomater. 2019;97:230–8.
Zheng M, Wang Z, Chang H, Wang L, Chew SWT, Lio DCS, Cui M, Liu L, Tee BCK, Xu C. Osmosis-powered hydrogel microneedles for microliters of skin interstitial fluid extraction within minutes. Adv Healthcare Mater. 2020;9(10):1901683.
Gerstel MS, Place VA. Drug delivery device, United States Patent, NO. 3964482.
Jin X, Zhu DD, Chen BZ, Ashfaq M, Guo XD. Insulin delivery systems combined with microneedle technology. Adv Drug Delivery Rev. 2018;127:119–37.
Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–9.
Singh S, Singh J. Transdermal drug delivery by passive diffusion and iontophoresis: a review. Med Res Rev. 1993;13(5):569–621.
Bliss M. The history of insulin. Diabetes Care. 1993;16(Suppl. 3):4–7.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl. 1):S98–110.
Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–17.
Donnelly RF, Singh TRR, Woolfson AD. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Delivery. 2010;17(4):187–207.
Richter-Johnson J, Kumar P, Choonara YE, du Toit LC, Pillay V. Therapeutic applications and pharmacoeconomics of microneedle technology. Expert Rev Pharmacoecon Outcomes Res. 2018;18(4):359–69.
Caffarel-Salvador E, Kim S, Soares V, Tian RY, Stern SR, Minahan D, Yona R, Lu X, Zakaria FR, Collins J, Wainer J, Wong J, McManus R, Tamang S, McDonnell S, Ishida K, Hayward A, Liu X, Hubalek F, Fels J, Vegge A, Frederiksen MR, Rahbek U, Yoshitake T, Fujimoto J, Roxhed N, Langer R, Traverso G. A microneedle platform for buccal macromolecule delivery. Sci Adv. 2021;7(4):eabe2620.
American Diabetes Association. Standards of medical care in diabetes-2020 abridged for primary care providers. Clinical diabetes: a publication of the American Diabetes Association. 2020;38(Suppl. 1):10–38.
Zong QD, Guo RR, Dong NJ, Ling GX, Zhang P. Design and development of insulin microneedles for diabetes treatment. Drug Delivery Transl Res. 2021.
Zhu M, Liu Y, Jiang F, Cao J, Kundu SC, Lu S. Combined silk fibroin microneedles for insulin delivery. ACS Biomater Sci Eng. 2020;6(6):3422–9.
Vora LK, Courtenay AJ, Tekko IA, Larraneta E, Donnelly RF. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int J Biol Macromol. 2020;146:290–8.
Ito Y, Yamazaki T, Sugioka N, Takada K. Self-dissolving micropile array tips for percutaneous administration of insulin. J Mater Sci: Mater Med. 2010;21(2):835–41.
Chen BZ, Ashfaq M, Zhu DD, Zhang XP, Guo XD. Controlled delivery of insulin using rapidly separating microneedles fabricated from genipin-crosslinked gelatin. Macromol Rapid Commun. 2018;39(20):1800075.
McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100(24):13755–60.
Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52(5):909–15.
Liu GJ, Yang ZG, Liu JF, Li XB, Wang H, Zhao T, Yang XL. A low cost, high performance insulin delivery system based on PZT actuation. Microsyst Technol. 2014;20(12):2287–94.
Ma B, Liu S, Gan Z, Liu G, Cai X, Zhang H, Yang Z. A PZT insulin pump integrated with a silicon microneedle array for transdermal drug delivery. Microfluid Nanofluid. 2006;2(5):417–23.
Meshkinfam F, Rizvi G. A MEMS based drug delivery device with integrated micro-needle array-design and simulation. J Biomech Eng. 2021;143(8):081010.
Rao KS, Sateesh J, Guha K, Baishnab KL, Ashok P, Sravani KG. Design and analysis of MEMS based piezoelectric micro pump integrated with micro needle. Microsyst Technol. 2020;26(10):3153–9.
Haldkar RK, Gupta VK, Sheorey T. Modeling and flow analysis of piezoelectric based micropump with various shapes of microneedle. J Mech Sci Technol. 2017;31(6):2933–41.
Yeung C, Chen S, King B, Lin H, King K, Akhtar F, Diaz G, Wang B, Zhu J, Sun W, Khademhosseini A, Emaminejad S. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics. 2019;13(6):064125.
DeMello J, DeMello A. Microscale reactors: Nanoscale products. Lab Chip. 2004;4(2):11N-N15.
Karnik R, Gu F, Basto P, Cannizzaro C, Dean L, Kyei-Manu W, Langer R, Farokhzad OC. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008;8(9):2906–12.
Khanna P, Strom JA, Malone JI, Bhansali S. Microneedle-based automated therapy for diabetes mellitus. J Diabetes Sci Technol. 2008;2(6):1122–9.
Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–40.
Takeuchi K, Takama N, Kim B, Sharma K, Paul O, Ruther P. Microfluidic chip to interface porous microneedles for ISF collection. Biomed Microdevices. 2019;21(1):28.
Li CG, Joung HA, Noh H, Song MB, Kim MG, Jung H. One-touch-activated blood multidiagnostic system using a minimally invasive hollow microneedle integrated with a paper-based sensor. Lab Chip. 2015;15(16):3286–92.
Ashraf MW, Tayyaba S, Nisar A, Afzulpurkar N. Fabrication and analysis of hollow microneedles and polymeric piezoelectric valveless micropump for transdermal drug-delivery system. IET Commun. 2012;6(18):3248–56.
Nordquist L, Roxhed N, Griss P, Stemme G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration. Pharm Res. 2007;24(7):1381–8.
Strambini LM, Longo A, Diligenti A, Barillaro G. A minimally invasive microchip for transdermal injection/sampling applications. Lab Chip. 2012;12(18):3370–9.
Mishra R, Maiti TK, Bhattacharyya TK. Feasibility studies on nafion membrane actuated micropump integrated with hollow microneedles for insulin delivery device. J Microelectromech Syst. 2019;28(6):987–96.
Mishra R, Pramanick B, Maiti TK, Bhatracharyya TK. Glassy carbon microneedles-new transdermal drug delivery device derived from a scalable C-MEMS process. Microsyst Nanoeng. 2018;4:38.
Economidou SN, Uddin MJ, Marques MJ, Douroumis D, Sow WT, Li H, Reid A, Windmill JFC, Podoleanu A. A novel 3D printed hollow microneedle microelectromechanical system for controlled, personalized transdermal drug delivery. Addit Manuf. 2021;38:101815.
Moussi K, Bukhamsin A, Hidalgo T, Kosel J. Biocompatible 3D printed microneedles for transdermal, intradermal, and percutaneous applications. Adv Eng Mater. 2020;22(2):1901358.
Trautmann A, Roth GL, Nujiqi B, Walther T, Hellmann R. Towards a versatile point-of-care system combining femtosecond laser generated microfluidic channels and direct laser written microneedle arrays. Microsyst Nanoeng. 2019;5:6.
American Diabetes Association. 7. Diabetes technology: Standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl. 1):S77–88.
Invernale MA, Tang BC, York RL, Le L, Hou DY, Anderson DG. Microneedle electrodes toward an amperometric glucosesensing smart patch. Adv Healthcare Mater. 2014;3(3):338–42.
Li X, Huang X, Mo J, Wang H, Huang Q, Yang C, Zhang T, Chen H-J, Hang T, Liu F, Jiang L, Wu Q, Li H, Hu N, Xie X. A fully integrated closed-loop system based on mesoporous microneedles-iontophoresis for diabetes treatment. Adv Sci. 2021;8(16).
Heifler O, Borberg E, Harpak N, Zverzhinetsky M, Krivitsky V, Gabriel I, Fourman V, Sherman D, Patolsky F. Clinic-on-a-needle array toward future minimally invasive wearable artificial pancreas applications. ACS Nano. 2021;15(7):12019–33.
Kim H, Theogarajan LS, Pennathur S. A repeatable and scalable fabrication method for sharp, hollow silicon microneedles. J Micromech Microeng. 2018;28(3):035007.
Gardeniers H, Luttge R, Berenschot EJW, de Boer MJ, Yeshurun SY, Hefetz M, Van’t Oever R, van den Berg A. Silicon micromachined hollow microneedles for transdermal liquid transport. J Microelectromech Syst. 2003;12(6):855–62.
Stoeber B, Liepmann D. Arrays of hollow out-of-plane microneedles for drug delivery. J Microelectromech Syst. 2005;14(3):472–9.
Ovsianikov A, Chichkov B, Mente P, Monteiro-Riviere NA, Doraiswamy A, Narayan RJ. Two photon polymerization of polymer-ceramic hybrid materials for transdermal drug delivery. Int J Appl Ceram Technol. 2007;4(1):22–9.
Laurent PE, Bourhy H, Fantino M, Alchas P, Mikszta JA. Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine. 2010;28(36):5850–6.
Velten T, Ruf HH, Barrow D, Aspragathos N, Lazarou P, Jung E, Malek CK, Richter M, Kruckow J. Packaging of bio-MEMS: Strategies, technologies, and applications. IEEE Trans Adv Packag. 2005;28(4):533–46.
Liu Y, Eng PF, Guy OJ, Roberts K, Ashraf H, Knight N. Advanced deep reactive-ion etching technology for hollow microneedles for transdermal blood sampling and drug delivery. IET Nanobiotechnol. 2013;7(2):59–62.
Strambini LM, Longo A, Scarano S, Prescimone T, Palchetti I, Minunni M, Giannessi D, Barillaro G. Self-powered microneedle-based biosensors for pain-free high-accuracy measurement of glycaemia in interstitial fluid. Biosens Bioelectron. 2015;66:162–8.
Zhu J, Zhou X, Libanori A, Sun W. Microneedle-based bioassays. Nanoscale Adv. 2020;2(10):4295–304.
Yang J, Li Y, Ye R, Zheng Y, Li X, Chen Y, Xie X, Jiang L. Smartphone-powered iontophoresis-microneedle array patch for controlled transdermal delivery. Microsyst Nanoeng. 2020;6(1).
Grayson ACR, Shawgo RS, Johnson AM, Flynn NT, Li YW, Cima MJ, Langer R. A BioMEMS review: MEMS technology for physiologically integrated devices. Proc IEEE. 2004;92(1):6–21.
Jina A, Tierney MJ, Tamada JA, McGill S, Desai S, Chua B, Chang A, Christiansen M. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J Diabetes Sci Technol. 2014;8(3):483–7.
Nakanishi N, Yamamoto H, Tsuchiya K, Uetsuji Y, Nakamachi E, Development of wearable medical device for bio-MEMS. In: Nicolau DV, editor., BioMEMS and Nanotechnology II. Brisbane; 2006. p. 60390P.
Yu J, Qian C, Zhang Y, Cui Z, Zhu Y, Shen Q, Ligler FS, Buse JB, Gu Z. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 2017;17(2):733–9.
Bankar SB, Bule MV, Singhal RS, Ananthanarayan L. Glucose oxidase — an overview. Biotechnol Adv. 2009;27(4):489–501.
Wu Q, Wang L, Yu HJ, Wang JJ, Chen ZF. Organization of glucose-responsive systems and their properties. Chem Rev. 2011;111(12):7855–75.
Steiner M-S, Duerkop A, Wolfbeis OS. Optical methods for sensing glucose. Chem Soc Rev. 2011;40(9):4805–39.
Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43(10):3595–629.
Hu X, Yu J, Qian C, Lu Y, Kahkoska AR, Xie Z, Jing X, Buse JB, Gu Z. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano. 2017;11(1):613–20.
Joshi-Barr S, Lux CD, Mahmoud E, Almutairi A. Exploiting oxidative microenvironments in the body as triggers for drug delivery systems. Antioxid Redox Signal. 2014;21(5):730–54.
Lux CdG, Joshi-Barr S, Trung N, Mahmoud E, Schopf E, Fomina N, Almutairi A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J Am Chem Soc. 2012;134(38):15758–64.
Wang J, Ye Y, Yu J, Kahkoska AR, Zhang X, Wang C, Sun W, Corder RD, Chen Z, Khan SA, Buse JB, Gu Z. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano. 2018;12(3):2466–73.
Gordijo CR, Shuhendler AJ, Wu XY. Glucose-responsive bioinorganic nanohybrid membrane for self-regulated insulin release. Adv Funct Mater. 2010;20(9):1404–12.
Wang Y, Cheng S, Hu W, Lin X, Cao C, Zou S, Tong Z, Jiang G, Kong X. Polymer-grafted hollow mesoporous silica nanoparticles integrated with microneedle patches for glucose-responsive drug delivery. Front Mater Sci. 2021;15(1):98–112.
Chen S, Matsumoto H, Moro-oka Y, Tanaka M, Miyahara Y, Suganami T, Matsumoto A. Microneedle-array patch fabricated with enzyme-free polymeric components capable of on-demand insulin delivery. Adv Funct Mater. 2019;29(7):1807369.
Zhang Y, Wang J, Yu J, Wen D, Kahkoska AR, Lu Y, Zhang X, Buse JB, Gu Z. Bioresponsive microneedles with a sheath structure for H2O2 and pH cascade-triggered insulin delivery. Small. 2018;14(14):1704181.
Xu B, Cao Q, Zhang Y, Yu W, Zhu J, Liu D, Jiang G. Microneedles integrated with ZnO quantum-dot-capped mesoporous bioactive glasses for glucose-mediated insulin delivery. ACS Biomater Sci Eng. 2018;4(7):2473–83.
Tong Z, Zhou J, Zhong J, Tang Q, Lei Z, Luo H, Ma P, Liu X. Glucose- and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats. ACS Appl Mater Interfaces. 2018;10(23):20014–24.
Xu B, Jiang G, Yu W, Liu D, Zhang Y, Zhou J, Sun S, Liu Y. H2O2-Responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J Mater Chem B. 2017;5(41):8200–8.
Ye Y, Yu J, Wang C, Nguyen N-Y, Walker GM, Buse JB, Gu Z. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv Mater. 2016;28(16):3115–21.
Yu JC, Zhang YQ, Ye YQ, DiSanto R, Sun WJ, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA. 2015;112(27):8260–5.
Lorand JP, Edwards JO. Polyol complexes and structure of the benzeneboronate ion. J Org Chem. 1959;24(6):769–74.
Preinerstorfer B, Laemmerhofer M, Lindner W. Synthesis and application of novel phenylboronate affinity materials based on organic polymer particles for selective trapping of glycoproteins. J Sep Sci. 2009;32(10):1673–85.
Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, Wang S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine. 2015;11(2):313–27.
Croissant JG, Fatieiev Y, Khashab NM. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv Mater. 2017;29(9):1604634.
Croissant JG, Fatieiev Y, Almalik A, Khashab NM. Mesoporous silica and organosilica nanoparticles: physical chemistry, biosafety, delivery strategies, and biomedical applications. Adv Healthcare Mater. 2018;7(4):1700831.
Wang Z, Wang J, Li H, Yu J, Chen G, Kahkoska AR, Wu V, Zeng Y, Wen D, Miedema JR, Buse JB, Gu Z. Dual self-regulated delivery of insulin and glucagon by a hybrid patch. Proc Natl Acad Sci USA. 2020;117(47):29512–7.
Chen F, Yan Q, Yu Y, Wu MX. BCG vaccine powder-laden and dissolvable microneedle arrays for lesion-free vaccination. J Controlled Release. 2017;255:36–44.
Kim S, Eum J, Yang H, Jung H. Transdermal finasteride delivery via powder-carrying microneedles with a diffusion enhancer to treat androgenetic alopecia. J Controlled Release. 2019;316:1–11.
Kim S, Yang H, Eum J, Ma Y, Lahiji SF, Jung H. Implantable powder-carrying microneedles for transdermal delivery of high-dose insulin with enhanced activity. Biomaterials. 2020;232:119733.
Donnelly RF, Singh TRR, Tunney MM, Morrow DIJ, McCarron PA, O’Mahony C, Woolfson AD. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26(11):2513–22.
Funding
This work was financially supported by the National Natural Science Foundation of China (51873015), the Joint Project of BRC-BC (Biomedical Translational Engineering Research Center of BUCT-CJFH) (XK2020-05, RZ2020-01), and the long-term subsidy mechanism from the Ministry of Finance and the Ministry of Education of PRC.
Author information
Authors and Affiliations
Contributions
Wen Xuan Li, Xiao Peng Zhang, Bo Zhi Chen, and Wen Min Fei: Writing — Original draft preparation. Yong Cui and Can Yang Zhang, Conceptualization. Xin Dong Guo: Conceptualization, Project administration.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from individual or guardian participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, W.X., Zhang, X.P., Chen, B.Z. et al. An update on microneedle-based systems for diabetes. Drug Deliv. and Transl. Res. 12, 2275–2286 (2022). https://doi.org/10.1007/s13346-021-01113-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01113-2