Abstract
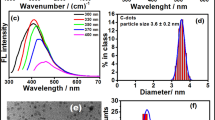
A lateral flow assay (LFA) sensor on a half-strip platform labeled with blue carbon nanodot-polystyrene (PS-CND) nanoconjugates was developed for the detection of carcinoembryonic antigen (CEA) levels in buffer and serum solutions from healthy and cancer patients. CNDs, biocompatible nanoparticles containing amino groups synthesized by hydrothermal synthesis, were conjugated to spherical polystyrene (PS) beads with an average diameter of 60 nm, followed by the attachment of a detection probe, anti-CEA (M0911042), using a heterobifunctional cross-linker. PS beads were used as a template in CND conjugates to provide uniform size and shape of fluorescent labels without losing CND fluorescence intensity after the antibody conjugation step and to improve fluorescence stability. Upon the interaction of CEA from samples with the anti-CEA (M0911042) probe-modified PS-CND, which was further adsorbed onto a test line composed of the capture anti-CEA (M0911041) physisorbed onto a nitrocellulose membrane, the fluorescent signals on the test line increased as a function of the CEA concentrations under irradiation with a portable 365 nm ultraviolet lamp. A linear concentration range of 0.04–70 nM in buffer was observed, with a limit of detection of 0.3 nM. The applicability of the developed LFA half-strip sensor for disease diagnosis was demonstrated by identifying fluorescent levels on the test line due to the presence of CEA in serum samples from cancer patients. Importantly, signals from healthy human serum solutions because of lower CEA concentrations beyond the sensor detection capability were clearly distinguished from the patient ones.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Ren, H.H., Jang, C.H.: A simple liquid crystal-based aptasensor using a hairpin-shaped aptamer for the bare-eye detection of carcinoembryonic antigen. Biochip J. 13, 352–361 (2019)
Hammarstrom, S.: The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 9, 67–81 (1999)
Arya, S.K., Bhansali, S.: Lung cancer and its early detection using biomarker-based biosensors. Chem. Rev. 111, 6783–6809 (2011)
Yang, G.J., et al.: Selective inhibition of lysine-specific demethylase 5A (KDM5A) using a rhodium(III) complex for triple-negative breast cancer therapy. Angew. Chem. Int. Ed. Engl. 57, 13091–13095 (2018)
Kumar, S., Mohan, A., Guleria, R.: Biomarkers in cancer screening, research and detection: present and future: a review. Biomarkers 11, 385–405 (2006)
Eom, G., et al.: Ultrasensitive detection of ovarian cancer biomarker using Au nanoplate SERS immunoassay. Biochip J. 15, 348–355 (2021)
Liu, L.J., et al.: Inhibition of the Ras/Raf interaction and repression of renal cancer xenografts in vivo by an enantiomeric iridium(III) metal-based compound. Chem. Sci. 8, 4756–4763 (2017)
Rusling, J.F., Kumar, C.V., Gutkind, J.S., Patel, V.: Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst 135, 2496–2511 (2010)
Yang, G., et al.: Recent advances in biosensor for detection of lung cancer biomarkers. Biosens. Bioelectron. 141, 111416 (2019)
Gao, N.L., Chang, J.G., Zhu, Z.M., You, H.: Multistory stairs-based, fast and point-of-care testing for disease biomarker using one-step capillary microfluidic fluoroimmunoassay chip via continuous on-chip labelling. Biochip J. 15, 268–275 (2021)
Park, M.: Orientation control of the molecular recognition layer for improved sensitivity: a review. Biochip J. 13, 82–94 (2019)
Jiang, N., et al.: Lateral and vertical flow assays for point-of-care diagnostics. Adv. Healthc. Mater. 8, e1900244 (2019)
Kim, S.K., Sung, H., Hwang, S.H., Kim, M.N.: A new quantum dot-based lateral flow immunoassay for the rapid detection of influenza viruses. Biochip J. 16, 1–8 (2022)
Bahadır, E.B., Sezgintürk, M.K.: Lateral flow assays: Principles, designs and labels. TRAC-Trend. Anal. Chem. 82, 286–306 (2016)
Parolo, C., et al.: Tutorial: design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 15, 3788–3816 (2020)
Nguyen, V.T., Song, S., Park, S., Joo, C.: Recent advances in high-sensitivity detection methods for paper-based lateral-flow assay. Biosens. Bioelectron. 152, 112015 (2020)
Mahmoudi, T., de la Guardia, M., Baradaran, B.: Lateral flow assays towards point-of-care cancer detection: a review of current progress and future trends. TRAC-Trend. Anal. Chem. 125, 115842 (2020)
Demchenko, A.P.: Photobleaching of organic fluorophores: quantitative characterization, mechanisms, protection. Methods Appl. Fluoresc. 8, 022001 (2020)
Valizadeh, A., et al.: Quantum dots: synthesis, bioapplications, and toxicity. Nanosc. Res. Lett. 7, 480 (2012)
Hardman, R.: A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 114, 165–172 (2006)
Li, H., Kang, Z., Liu, Y., Lee, S.-T.: Carbon nanodots: synthesis, properties and applications. J. Mater. Chem. 22, 24230–24253 (2012)
Zhu, S., et al.: Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. Engl. 52, 3953–3957 (2013)
Park, Y., Yoo, J., Lim, B., Kwon, W., Rhee, S.W.: Improving the functionality of carbon nanodots: doping and surface functionalization. J. Mater. Chem. A 4, 11582–11603 (2016)
Hsu, P.-C., Chang, H.-T.: Synthesis of high-quality carbon nanodots from hydrophilic compounds: role of functional groups. Chem. Commun. 48, 3984–3986 (2012)
Vedamalai, M., et al.: Carbon nanodots prepared from o-phenylenediamine for sensing of Cu2+ ions in cells. Nanoscale 6, 13119–13125 (2014)
Hu, Q., Gong, X.J., Liu, L.Z., Choi, M.M.F.: Characterization and analytical separation of fluorescent carbon nanodots. J. Nanomater. 12, 1–23 (2017)
Jin, K., et al.: Facile access to solid-state carbon dots with high luminescence efficiency and excellent formability via cellulose derivative coatings. ACS Sustain. Chem. Eng. 8, 5937–5945 (2020)
Li, M., Chen, T., Gooding, J.J., Liu, J.: Review of carbon and graphene quantum dots for sensing. ACS Sens. 4, 1732–1748 (2019)
Shi, B.B., et al.: Ultra-robust, highly proton-conductive polymer carbon dot membranes through bioinspired complexation. J. Mater. Chem. A 10, 16995–17000 (2022)
Supianto, M., et al.: Fluorescent paper strip immunoassay with carbon nanodots@silica for determination of human serum amyloid A1. Microchim. Acta 188, 386 (2021)
Goh, E., Lee, H.J.: Biofunctionalized carbon nanodot-polystyrene bead conjugates for bioanalysis applications. Bull. Korean Chem. Soc. 41, 776–777 (2020)
Lee, H. N. et al. A lateral flow assay for nucleic acid detection based on rolling circle amplification using capture ligand-modified oligonucleotides. Biochip J. 16, 441–450 (2022)
Jeon, J., et al.: Improvement of reproducibility and thermal stability of surface-enhanced Raman scattering-based lateral flow assay strips using silica-encapsulated gold nanoparticles. Sens. Actuators B Chem. 321, 128521 (2020)
Loynachan, C.N., et al.: Platinum nanocatalyst amplification: redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 12, 279–288 (2018)
Li, T., Zhou, C., Jiang, M.: UV absorption-spectra of polystyrene. Polym. Bull. 25, 211–216 (1991)
Zhang, Q., Wang, R., Feng, B., Zhong, X., Ostrikov, K.K.: Photoluminescence mechanism of carbon dots: triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 12, 6856 (2021)
Gole, A., Vyas, S., Phadtare, S., Lachke, A., Sastry, M.: Studies on the formation of bioconjugates of Endoglucanase with colloidal gold. Colloids Surf. B 25, 129–138 (2002)
Wang, J., et al.: Ratiometric fluorescent lateral flow immunoassay for point-of-care testing of acute myocardial infarction. Angew. Chem. Int. Ed. Engl. 60, 13042–13049 (2021)
Li, H., Han, D., Hegener, M.A., Pauletti, G.M., Steckl, A.J.: Flow reproducibility of whole blood and other bodily fluids in simplified no reaction lateral flow assay devices. Biomicrofluidics 11, 024116 (2017)
Kainz, D.M., et al.: Eliminating viscosity bias in lateral flow tests. Microsyst. Nanoeng. 7, 72 (2021)
Galush, W.J., Le, L.N., Moore, J.M.: Viscosity behavior of high-concentration protein mixtures. J. Pharm. Sci. 101, 1012–1020 (2012)
Christopoulou, N.M., Kalogianni, D.P., Christopoulos, T.K.: Macromolecular crowding agents enhance the sensitivity of lateral flow immunoassays. Biosens. Bioelectron. 218, 114737 (2022)
Rivas, G., Minton, A.P.: Macromolecular crowding in vitro, in vivo, and in between. Trends Biochem. Sci. 41, 970–981 (2016)
Zhang, S.-F., et al.: Sensitivity enhancement of lateral flow assay by embedding cotton threads in paper. Cellulose 26, 8087–8099 (2019)
Liu, C., et al.: Lateral flow immunochromatographic assay for sensitive pesticide detection by using Fe3O4 nanoparticle aggregates as color reagents. Anal. Chem. 83, 6778–6784 (2011)
He, X., et al.: Sensitivity enhancement of nucleic acid lateral flow assays through a physical-chemical coupling method: dissoluble saline barriers. ACS Sens. 4, 1691–1700 (2019)
Kaur, M., Eltzov, E.: Optimizing effective parameters to enhance the sensitivity of vertical flow assay for detection of Escherichia coli. Biosensors 12, 63 (2022)
Park, S.B., Shin, J.H.: Pressed lateral flow assay strips for flow delay-induced signal enhancement in lateral flow assay strips. Biochip J. 16, 480–489 (2022)
Grunnet, M., Sorensen, J.B.: Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 76, 138–143 (2012)
Hine, K.R., Dykes, P.W.: Serum CEA testing in the post-operative surveillance of colorectal carcinoma. Br. J. Cancer 49, 689–693 (1984)
Icard, P., et al.: Preoperative carcinoembryonic antigen level as a prognostic indicator in resected primary lung-cancer. Ann. Thorac. Surg. 58, 811–814 (1994)
de Puig, H., Bosch, I., Carre-Camps, M., Hamad-Schifferli, K.: Effect of the protein corona on antibody-antigen binding in nanoparticle sandwich immunoassays. Bioconjug. Chem. 28, 230–238 (2017)
Huhn, D., et al.: Polymer-coated nanoparticles interacting with proteins and cells: focusing on the sign of the net charge. ACS Nano 7, 3253–3263 (2013)
Rocker, C., Potzl, M., Zhang, F., Parak, W.J., Nienhaus, G.U.: A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat. Nanotechnol. 4, 577–580 (2009)
Ritz, S., et al.: Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromol 16, 1311–1321 (2015)
Mathios, D., et al.: Detection and characterization of lung cancer using cell-free DNA fragmentomes. Nat. Commun. 12, 5060 (2021)
Acknowledgements
This study was supported by a National Research Foundation of Korea (NRF) grant, funded by the Korean government (Ministry of Science and ICT, MSIT) (grant numbers NRF-2020R1A4A1018393) and Kick the hurdle internal research fund (KTHD-R-001). All biospecimens and data used in this study were provided by the Biobank of Korea-Kyungpook National University Hospital (KNUH), a member of the Korea Biobank Network. All materials derived from the National Biobank of Korea-KNUH were obtained (with informed consent) under Institutional Review Board (IRB)-approved protocols.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim, J.M., Supianto, M., Kim, T.Y. et al. Fluorescent Lateral Flow Assay with Carbon Nanodot Conjugates for Carcinoembryonic Antigen. BioChip J 17, 93–103 (2023). https://doi.org/10.1007/s13206-022-00093-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-022-00093-w