Abstract
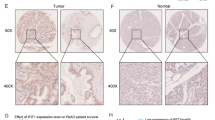
The tumor susceptibility gene 101 (TSG101) has been reported to play important roles in the development and progression of several human cancers, such as pancreatic cancer, prostate cancer, and hepatocellular carcinoma. However, its potential roles and underlined mechanisms in human glioma are still needed to be further clarified. This study was designed to assess the expression of TSG101 in glioma patients and its effects on glioma cell proliferation, migration, and invasion. Publicly available data revealed that TSG101 mRNA was significantly upregulated in glioma tissues, and high levels of TSG101 were associated with poor prognosis in glioma patients. Western blot and immunohistochemistry experiments further showed that the expression level of TSG101 protein was significantly upregulated in glioma patients, especially in the patients with high-grade glioma. The functional studies showed that knockdown of TSG101 suppressed the proliferation, migration, and invasion of glioma cells, while overexpression of TSG101 facilitated them. Mechanistic studies indicated that the proliferation, migration, and invasion induced by TSG101 in human glioma were related to AKT/GSK3β/β-catenin and RhoC/Cofilin signaling pathways. In conclusion, the above results suggest that the expression of TSG101 is elevated in glioma patients, which accelerates the proliferation, migration, and invasion of glioma cells by regulating the AKT/GSK3β/β-catenin and RhoC/Cofilin pathways.







Similar content being viewed by others
Data Availability
The datasets supporting the conclusions of this article are included within the article and its additional files.
References
Ostrom QT, Gittleman H, Stetson L, Virk SM, Barnholtz-Sloan JS (2015) Epidemiology of gliomas. Cancer Treat Res 163:1–14. https://doi.org/10.1007/978-3-319-12048-5_1
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820. https://doi.org/10.1007/s00401-016-1545-1
Shahar T, Rozovski U, Hess KR, Hossain A, Gumin J, Gao F, Fuller GN, Goodman L et al (2017) Percentage of mesenchymal stem cells in high-grade glioma tumor samples correlates with patient survival. Neuro-Oncology 19(5):660–668. https://doi.org/10.1093/neuonc/now239
Li L, Cohen SN (1996) Tsg101: A novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell 85(3):319–329
Ponting CP, Cai YD, Bork P (1997) The breast cancer gene product TSG101: a regulator of ubiquitination? J Mol Med (Berl) 75(7):467–469
Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH, Wang HE, Wettstein DA, Stray KM et al (2001) Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107(1):55–65
Koonin EV, Abagyan RA (1997) TSG101 may be the prototype of a class of dominant negative ubiquitin regulators. Nat Genet 16(4):330–331. https://doi.org/10.1038/ng0897-330
Carstens MJ, Krempler A, Triplett AA, Van Lohuizen M, Wagner KU (2004) Cell cycle arrest and cell death are controlled by p53-dependent and p53-independent mechanisms in Tsg101-deficient cells. J Biol Chem 279(34):35984–35994. https://doi.org/10.1074/jbc.M400408200
Amit I, Yakir L, Katz M, Zwang Y, Marmor MD, Citri A, Shtiegman K, Alroy I et al (2004) Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding. Genes Dev 18(14):1737–1752. https://doi.org/10.1101/gad.294904
McDonald B, Martin-Serrano J (2008) Regulation of Tsg101 expression by the steadiness box: a role of Tsg101-associated ligase. Mol Biol Cell 19(2):754–763. https://doi.org/10.1091/mbc.e07-09-0957
Majumder P, Chakrabarti O (2015) Mahogunin regulates fusion between amphisomes/MVBs and lysosomes via ubiquitination of TSG101. Cell Death Dis 6:e1970. https://doi.org/10.1038/cddis.2015.257
Jiao J, Sun K, Walker WP, Bagher P, Cota CD, Gunn TM (2009) Abnormal regulation of TSG101 in mice with spongiform neurodegeneration. Biochim Biophys Acta 1792(10):1027–1035. https://doi.org/10.1016/j.bbadis.2009.08.009
Cheng TH, Cohen SN (2007) Human MDM2 isoforms translated differentially on constitutive versus p53-regulated transcripts have distinct functions in the p53/MDM2 and TSG101/MDM2 feedback control loops. Mol Cell Biol 27(1):111–119. https://doi.org/10.1128/MCB.00235-06
Li L, Liao J, Ruland J, Mak TW, Cohen SN (2001) A TSG101/MDM2 regulatory loop modulates MDM2 degradation and MDM2/p53 feedback control. Proc Natl Acad Sci U S A 98(4):1619–1624. https://doi.org/10.1073/pnas.98.4.1619
Young TW, Rosen DG, Mei FC, Li N, Liu J, Wang XF, Cheng X (2007) Up-regulation of tumor susceptibility gene 101 conveys poor prognosis through suppression of p21 expression in ovarian cancer. Clin Cancer Res 13(13):3848–3854. https://doi.org/10.1158/1078-0432.CCR-07-0337
Ma XR, Edmund Sim UH, Pauline B, Patricia L, Rahman J (2008) Overexpression of WNT2 and TSG101 genes in colorectal carcinoma. Trop Biomed 25(1):46–57
Liu RT, Huang CC, You HL, Chou FF, Hu CC, Chao FP, Chen CM, Cheng JT (2002) Overexpression of tumor susceptibility gene TSG101 in human papillary thyroid carcinomas. Oncogene 21(31):4830–4837. https://doi.org/10.1038/sj.onc.1205612
Koon N, Schneider-Stock R, Sarlomo-Rikala M, Lasota J, Smolkin M, Petroni G, Zaika A, Boltze C et al (2004) Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut 53(2):235–240
Liu DC, Yang ZL, Jiang S (2011) Identification of PEG10 and TSG101 as carcinogenesis, progression, and poor-prognosis related biomarkers for gallbladder adenocarcinoma. Pathol Oncol Res 17(4):859–866. https://doi.org/10.1007/s12253-011-9394-7
Liu Z, Tian Z, Cao K, Zhang B, Wen Q, Zhou X, Yang W, Wang T et al (2019) TSG101 promotes the proliferation, migration and invasion of hepatocellular carcinoma cells by regulating the PEG10. J Cell Mol Med 23(1):70–82. https://doi.org/10.1111/jcmm.13878
Chua HH, Kameyama T, Mayeda A, Yeh TH (2019) Cancer-specifically re-spliced TSG101 mRNA promotes invasion and metastasis of nasopharyngeal carcinoma. Int J Mol Sci 20(3). https://doi.org/10.3390/ijms20030773
Shao Z, Ji W, Liu A, Qin A, Shen L, Li G, Zhou Y, Hu X et al (2015) TSG101 silencing suppresses hepatocellular carcinoma cell growth by inducing cell cycle arrest and Autophagic cell death. Med Sci Monit 21:3371–3379. https://doi.org/10.12659/msm.894447
Suzuki Y, Shirai K, Oka K, Mobaraki A, Yoshida Y, Noda SE, Okamoto M, Suzuki Y et al (2010) Higher pAkt expression predicts a significant worse prognosis in glioblastomas. J Radiat Res 51(3):343–348
Xue L, Wang Y, Yue S, Zhang J (2015) Low MiR-149 expression is associated with unfavorable prognosis and enhanced Akt/mTOR signaling in glioma. Int J Clin Exp Pathol 8(9):11178–11184
Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV (2005) The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene 24(50):7482–7492. https://doi.org/10.1038/sj.onc.1209088
ter Haar E, Coll JT, Austen DA, Hsiao HM, Swenson L, Jain J (2001) Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat Struct Biol 8(7):593–596. https://doi.org/10.1038/89624
Jope RS, Johnson GV (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29(2):95–102. https://doi.org/10.1016/j.tibs.2003.12.004
Atkins RJ, Stylli SS, Luwor RB, Kaye AH, Hovens CM (2013) Glycogen synthase kinase-3beta (GSK-3beta) and its dysregulation in glioblastoma multiforme. J Clin Neurosci 20(9):1185–1192. https://doi.org/10.1016/j.jocn.2013.02.003
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B et al (1998) Identification of c-MYC as a target of the APC pathway. Science 281(5382):1509–1512
Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ, Boman BM (2001) Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res 61(24):8664–8667
Shiina H, Igawa M, Breault J, Ribeiro-Filho L, Pookot D, Urakami S, Terashima M, Deguchi M et al (2003) The human T-cell factor-4 gene splicing isoforms, Wnt signal pathway, and apoptosis in renal cell carcinoma. Clin Cancer Res 9(6):2121–2132
Liu C, Tu Y, Sun X, Jiang J, Jin X, Bo X, Li Z, Bian A et al (2011) Wnt/beta-catenin pathway in human glioma: expression pattern and clinical/prognostic correlations. Clin Exp Med 11(2):105–112. https://doi.org/10.1007/s10238-010-0110-9
Sareddy GR, Panigrahi M, Challa S, Mahadevan A, Babu PP (2009) Activation of Wnt/beta-catenin/Tcf signaling pathway in human astrocytomas. Neurochem Int 55(5):307–317. https://doi.org/10.1016/j.neuint.2009.03.016
Wheeler AP, Ridley AJ (2004) Why three rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res 301(1):43–49. https://doi.org/10.1016/j.yexcr.2004.08.012
Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, Khokha R, Mak TW (2005) RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev 19(17):1974–1979. https://doi.org/10.1101/gad.1310805
Kleer CG, Griffith KA, Sabel MS, Gallagher G, van Golen KL, Wu ZF, Merajver SD (2005) RhoC-GTPase is a novel tissue biomarker associated with biologically aggressive carcinomas of the breast. Breast Cancer Res Treat 93(2):101–110. https://doi.org/10.1007/s10549-005-4170-6
Clark EA, Golub TR, Lander ES, Hynes RO (2000) Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406(6795):532–535. https://doi.org/10.1038/35020106
Matsuoka T, Yashiro M (2014) Rho/ROCK signaling in motility and metastasis of gastric cancer. World J Gastroenterol 20(38):13756–13766. https://doi.org/10.3748/wjg.v20.i38.13756
Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J (2011) A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol 21(8):635–644. https://doi.org/10.1016/j.cub.2011.03.039
Coumans JVF, Davey RJ, Moens PDJ (2018) Cofilin and profilin: partners in cancer aggressiveness. Biophys Rev 10(5):1323–1335. https://doi.org/10.1007/s12551-018-0445-0
Zou S, Zhu Y, Wang B, Qian F, Zhang X, Wang L, Fu C, Bao H et al (2017) The ubiquitin ligase COP1 promotes glioma cell proliferation by preferentially downregulating tumor suppressor p53. Mol Neurobiol 54(7):5008–5016. https://doi.org/10.1007/s12035-016-0033-x
Roy A, Ansari SA, Das K, Prasad R, Bhattacharya A, Mallik S, Mukherjee A, Sen P (2017) Coagulation factor VIIa-mediated protease-activated receptor 2 activation leads to beta-catenin accumulation via the AKT/GSK3beta pathway and contributes to breast cancer progression. J Biol Chem 292(33):13688–13701. https://doi.org/10.1074/jbc.M116.764670
Quintayo MA, Munro AF, Thomas J, Kunkler IH, Jack W, Kerr GR, Dixon JM, Chetty U et al (2012) GSK3beta and cyclin D1 expression predicts outcome in early breast cancer patients. Breast Cancer Res Treat 136(1):161–168. https://doi.org/10.1007/s10549-012-2229-8
Majeed R, Hussain A, Sangwan PL, Chinthakindi PK, Khan I, Sharma PR, Koul S, Saxena AK et al (2016) PI3K target based novel cyano derivative of betulinic acid induces its signalling inhibition by down-regulation of pGSK3beta and cyclin D1 and potentially checks cancer cell proliferation. Mol Carcinog 55(5):964–976. https://doi.org/10.1002/mc.22339
Liu Z, Yang Z, Liu D, Li D, Zou Q, Yuan Y, Li J, Liang L et al (2014) TSG101 and PEG10 are prognostic markers in squamous cell/adenosquamous carcinomas and adenocarcinoma of the gallbladder. Oncol Lett 7(4):1128–1138. https://doi.org/10.3892/ol.2014.1886
Zhu Y, Zhang X, Wang L, Ji Z, Xie M, Zhou X, Liu Z, Shi H et al (2017) Loss of SH3GL2 promotes the migration and invasion behaviours of glioblastoma cells through activating the STAT3/MMP2 signalling. J Cell Mol Med 21(11):2685–2694. https://doi.org/10.1111/jcmm.13184
Yu Z, Sun Y, She X, Wang Z, Chen S, Deng Z, Zhang Y, Liu Q et al (2017) SIX3, a tumor suppressor, inhibits astrocytoma tumorigenesis by transcriptional repression of AURKA/B. J Hematol Oncol 10(1):115. https://doi.org/10.1186/s13045-017-0483-2
Funding
This work was supported by the National Natural Science Foundation of China (81672490 and 81874081), the Natural Science Foundation of Jiangsu Province of China (BK20181149), the Foundation of Xuzhou Science and Technology Bureau (KC20139), the Jiangsu Provincial Qing Lan Project and Jiangsu Provincial Medical Youth Talent (QNRC2016784), and the Young Science and Technology Innovation Team of Xuzhou Medical University Key Research and Development Plan of Jiangsu Province (TD202006).
Author information
Authors and Affiliations
Contributions
YZ and YX performed the experimental procedures. TC and YZ constructed the plasmids. QM, SR, and LW assisted in the data analysis. HS designed and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to Participate
The experimental protocol for animal studies was reviewed and approved by the Ethics Committee of Xuzhou Medical University. Human glioma tissues were obtained from the Affiliated Hospital of Xuzhou Medical University. This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 5061 kb)
Rights and permissions
About this article
Cite this article
Zhu, Y., Xu, Y., Chen, T. et al. TSG101 Promotes the Proliferation, Migration, and Invasion of Human Glioma Cells by Regulating the AKT/GSK3β/β-Catenin and RhoC/Cofilin Pathways. Mol Neurobiol 58, 2118–2132 (2021). https://doi.org/10.1007/s12035-020-02231-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02231-7