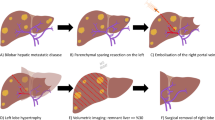
Abstract
Purpose
Nearly one-third of patients diagnosed with colorectal cancer (CRC) will ultimately develop metastatic disease. While a small percentage of patients can be considered for curative resection, more patients have limited disease that can be considered for local therapy. Challenges remain in defining oligometastatic CRC as well as developing treatment strategies guided by high level evidence.
Methods
In this review, we present the challenges in defining oligometastatic CRC and summarize the current literature on treatment and outcomes of local therapy in patients with metastatic CRC.
Results
For patients with liver- and/or lung-confined CRC metastases, surgical resection is the standard of care given the potential for long-term progression-free and overall survival. For patients with liver- or lung-confined disease not amenable to surgical resection, non-surgical local therapies, such as thermal ablation, hepatic arterial infusion pump (HAIP), or stereotactic body radiation therapy (SBRT), should be considered. For patients with more advanced disease, such as lymph node or bony metastases, the role of metastasis-directed therapy is controversial. Emerging data suggests that SBRT to ablate all metastases can improve progression-free and overall survival.
Conclusion
Multidisciplinary management is critical for patients with metastatic CRC due to the complexity of their cases and the nuanced patient, tumor, biological, and anatomical factors that must be weighed when considering local therapy. High-quality prospective randomized data in CRC are needed to further clarify the role of local ablative therapy in patients with unresectable oligometastatic CRC with ongoing studies including the RESOLUTE trial (ACTRN12621001198819) and the upcoming NCTN ERASur trial (NCT05673148).
Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. https://doi.org/10.3322/caac.21708.
Elferink MA, De Jong KP, Klaase JM, Siemerink EJ, De Wilt JH. Metachronous metastases from colorectal cancer: a population-based study in North-East Netherlands. Int J Colorectal Dis. 2015;30:205–12. https://doi.org/10.1007/s00384-014-2085-6.
Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. https://doi.org/10.1038/srep29765.
deSouza NM, Tempany CM. A risk-based approach to identifying oligometastatic disease on imaging. Int J Cancer. 2019;144:422–30. https://doi.org/10.1002/ijc.31793.
Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–66. https://doi.org/10.1016/j.radonc.2020.04.003.
Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, De Souza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–28. https://doi.org/10.1016/S1470-2045(19)30718-1.
Pitroda SP, Khodarev NN, Huang L, Uppal A, Wightman SC, Ganai S, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018;9:1793. https://doi.org/10.1038/s41467-018-04278-6.
Choti MA, Thomas M, Wong SL, Eaddy M, Pawlik TM, Hirose K, et al. Surgical resection preferences and perceptions among medical oncologists treating liver metastases from colorectal cancer. Ann Surg Oncol. 2016;23:375–81. https://doi.org/10.1245/s10434-015-4925-1.
Morris VK, Kennedy EB, Baxter NN, Benson AB, 3rd, Cercek A, Cho M, et al. Treatment of metastatic colorectal cancer: ASCO Guideline. J Clin Oncol. 2022;JCO2201690. https://doi.org/10.1200/JCO.22.01690
Lowes M, Kleiss M, Lueck R, Detken S, Koenig A, Nietert M, et al. The utilization of multidisciplinary tumor boards (MDT) in clinical routine: results of a health care research study focusing on patients with metastasized colorectal cancer. Int J Colorectal Dis. 2017;32:1463–9. https://doi.org/10.1007/s00384-017-2871-z.
Adam R, Vinet E. Regional treatment of metastasis: surgery of colorectal liver metastases. Ann Oncol. 2004;15(Suppl 4):iv103–6. https://doi.org/10.1093/annonc/mdh912
Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. https://doi.org/10.1007/BF00316981.
Butler J, Attiyeh FF, Daly JM. Hepatic resection for metastases of the colon and rectum. Surg Gynecol Obstet. 1986;162:109–13.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–15. https://doi.org/10.1016/S1470-2045(13)70447-9.
Kanemitsu Y, Shimizu Y, Mizusawa J, Inaba Y, Hamaguchi T, Shida D, et al. Hepatectomy followed by mFOLFOX6 versus hepatectomy alone for liver-only metastatic colorectal cancer (JCOG0603): a phase II or III randomized controlled trial. J Clin Oncol. 2021;39:3789–99. https://doi.org/10.1200/JCO.21.01032.
Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13:668–76. https://doi.org/10.1245/ASO.2006.05.039.
Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–80. https://doi.org/10.1200/JCO.2007.11.0833.
Chavez MI, Gholami S, Kim BJ, Margonis GA, Ethun CG, Tsai S, et al. Two-stage hepatectomy for bilateral colorectal liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2021;28:1457–65. https://doi.org/10.1245/s10434-020-09459-6.
Mokdad A, Choti MA, Yopp AC. Conversion chemotherapy for unresectable colorectal liver metastases: are we making a difference? Curr Colorect Canc R. 2015;11:160–7. https://doi.org/10.1007/s11888-015-0271-8.
Barone C, Nuzzo G, Cassano A, Basso M, Schinzari G, Giuliante F, et al. Final analysis of colorectal cancer patients treated with irinotecan and 5-fluorouracil plus folinic acid neoadjuvant chemotherapy for unresectable liver metastases. Br J Cancer. 2007;97:1035–9. https://doi.org/10.1038/sj.bjc.6603988.
Masi G, Loupakis F, Pollina L, Vasile E, Cupini S, Ricci S, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249:420–5. https://doi.org/10.1097/SLA.0b013e31819a0486.
Ychou M, Rivoire M, Thezenas S, Guimbaud R, Ghiringhelli F, Mercier-Blas A, et al. Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial. Br J Cancer. 2022;126:1264–70. https://doi.org/10.1038/s41416-021-01644-y.
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31:1931–8. https://doi.org/10.1200/JCO.2012.44.8308.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–16. https://doi.org/10.1016/S0140-6736(08)60455-9.
Sonbol MB, Siddiqi R, Uson PLS, Pathak S, Firwana B, Botrus G, et al. The role of systemic therapy in resectable colorectal liver metastases: systematic review and network meta-analysis. Oncologist. 2022. https://doi.org/10.1093/oncolo/oyac212.
Boudjema K, Locher C, Sabbagh C, Ortega-Deballon P, Heyd B, Bachellier P, et al. Simultaneous versus delayed resection for initially resectable synchronous colorectal cancer liver metastases: a prospective, open-label, randomized, controlled trial. Ann Surg. 2021;273:49–56. https://doi.org/10.1097/SLA.0000000000003848.
Feng Q, Wei Y, Zhu D, Ye L, Lin Q, Li W, et al. Timing of hepatectomy for resectable synchronous colorectal liver metastases: for whom simultaneous resection is more suitable—a meta-analysis. PLoS One. 2014;9:e104348. https://doi.org/10.1371/journal.pone.0104348.
Takamoto T, Hashimoto T, Miyata A, Shimada K, Maruyama Y, Makuuchi M. Repeat hepatectomy after major hepatectomy for colorectal liver metastases. J Gastrointest Surg. 2020;24:380–7. https://doi.org/10.1007/s11605-019-04154-8.
Valenzuela CD, Moaven O, Gawdi R, Stauffer JA, Del Piccolo NR, Cheung TT, et al. Outcomes after repeat hepatectomy for colorectal liver metastases from the colorectal liver operative metastasis international collaborative (COLOMIC). J Surg Oncol. 2022. https://doi.org/10.1002/jso.27056.
Lin YM, Paolucci I, Brock KK, Odisio BC. Image-guided ablation for colorectal liver metastasis: principles, current evidence, and the path forward. Cancers (Basel). 2021;13. https://doi.org/10.3390/cancers13163926.
Ruers T, Punt C, Van Coevorden F, Pierie J, Borel-Rinkes I, Ledermann JA, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol. 2012;23:2619–26. https://doi.org/10.1093/annonc/mds053.
Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109. https://doi.org/10.1093/jnci/djx015.
Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192-203. https://doi.org/10.1016/j.jvir.2010.04.007.
Knott EA, Ziemlewicz TJ, Lubner SJ, Swietlik JF, Weber SM, Zlevor AM, et al. Microwave ablation for colorectal cancer metastasis to the liver: a single-center retrospective analysis. J Gastrointest Oncol. 2021;12:1454–69. https://doi.org/10.21037/jgo-21-159.
Shady W, Petre EN, Do KG, Gonen M, Yarmohammadi H, Brown KT, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(268–275):e1. https://doi.org/10.1016/j.jvir.2017.08.021
Puijk RS, Dijkstra M, van den Bemd BAT, Ruarus AH, Nieuwenhuizen S, Geboers B, et al. Improved outcomes of thermal ablation for colorectal liver metastases: a 10-year analysis from the prospective Amsterdam CORE Registry (AmCORE). Cardiovasc Intervent Radiol. 2022;45:1074–89. https://doi.org/10.1007/s00270-022-03152-9.
Mimmo A, Pegoraro F, Rhaiem R, Montalti R, Donadieu A, Tashkandi A, et al. Microwave ablation for colorectal liver metastases: a systematic review and pooled oncological analyses. Cancers (Basel). 2022;14. https://doi.org/10.3390/cancers14051305.
Joo JH, Park JH, Kim JC, Yu CS, Lim SB, Park IJ, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys. 2017;99:876–83. https://doi.org/10.1016/j.ijrobp.2017.07.030.
Scorsetti M, Comito T, Tozzi A, Navarria P, Fogliata A, Clerici E, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol. 2015;141:543–53. https://doi.org/10.1007/s00432-014-1833-x.
Petrelli F, Comito T, Barni S, Pancera G, Scorsetti M, Ghidini A, et al. Stereotactic body radiotherapy for colorectal cancer liver metastases: a systematic review. Radiother Oncol. 2018;129:427–34. https://doi.org/10.1016/j.radonc.2018.06.035.
Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983;10:176–82.
Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395–403. https://doi.org/10.1200/JCO.2005.03.8166.
Lorenz M, Muller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2000;18:243–54. https://doi.org/10.1200/JCO.2000.18.2.243.
Dhir M, Jones HL, Shuai Y, Clifford AK, Perkins S, Steve J, et al. Hepatic arterial infusion in combination with modern systemic chemotherapy is associated with improved survival compared with modern systemic chemotherapy alone in patients with isolated unresectable colorectal liver metastases: a case-control study. Ann Surg Oncol. 2017;24:150–8. https://doi.org/10.1245/s10434-016-5418-6.
Pak LM, Kemeny NE, Capanu M, Chou JF, Boucher T, Cercek A, et al. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: Long term results and curative potential. J Surg Oncol. 2018;117:634–43. https://doi.org/10.1002/jso.24898.
Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–77.
Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32:1387–95.
Martin RC 2nd, Scoggins CR, Schreeder M, Rilling WS, Laing CJ, Tatum CM, et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer. 2015;121:3649–58. https://doi.org/10.1002/cncr.29534.
Westcott MA, Coldwell DM, Liu DM, Zikria JF. The development, commercialization, and clinical context of yttrium-90 radiolabeled resin and glass microspheres. Adv Radiat Oncol. 2016;1:351–64. https://doi.org/10.1016/j.adro.2016.08.003.
Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18:1159–71. https://doi.org/10.1016/S1470-2045(17)30457-6.
Mulcahy MF, Mahvash A, Pracht M, Montazeri AH, Bandula S, Martin RCG 2nd, et al. Radioembolization with chemotherapy for colorectal liver metastases: a randomized, open-label, international, multicenter, phase III trial. J Clin Oncol. 2021;39:3897–907. https://doi.org/10.1200/JCO.21.01839.
Dhir M, Zenati MS, Jones HL, Bartlett DL, Choudry MHA, Pingpank JF, et al. Effectiveness of hepatic artery infusion (HAI) versus selective internal radiation therapy (Y90) for pretreated isolated unresectable colorectal liver metastases (IU-CRCLM). Ann Surg Oncol. 2018;25:550–7. https://doi.org/10.1245/s10434-017-6265-9.
Raphael MJ, Karanicolas PJ. Regional therapy for colorectal cancer liver metastases: which modality and when? J Clin Oncol. 2022;40:2806–17. https://doi.org/10.1200/JCO.21.02505.
Milosevic M, Edwards J, Tsang D, Dunning J, Shackcloth M, Batchelor T, et al. Pulmonary metastasectomy in colorectal cancer: updated analysis of 93 randomized patients – control survival is much better than previously assumed. Colorectal Dis. 2020;22:1314–24. https://doi.org/10.1111/codi.15113.
Booth CM, Nanji S, Wei X, Mackillop WJ. Outcomes of resected colorectal cancer lung metastases in routine clinical practice: a population-based study. Ann Surg Oncol. 2016;23:1057–63. https://doi.org/10.1245/s10434-015-4979-0.
Davini F, Ricciardi S, Zirafa CC, Romano G, Ali G, Fontanini G, et al. Lung metastasectomy after colorectal cancer: prognostic impact of resection margin on long term survival, a retrospective cohort study. Int J Colorectal Dis. 2020;35:9–18. https://doi.org/10.1007/s00384-019-03386-z.
Okumura T, Boku N, Hishida T, Ohde Y, Sakao Y, Yoshiya K, et al. Surgical outcome and prognostic stratification for pulmonary metastasis from colorectal cancer. Ann Thorac Surg. 2017;104:979–87. https://doi.org/10.1016/j.athoracsur.2017.03.021.
Patel D, Townsend AR, Karapetis C, Beeke C, Padbury R, Roy A, et al. Is survival for patients with resectable lung metastatic colorectal cancer comparable to those with resectable liver disease? Results from the South Australian Metastatic Colorectal Registry. Ann Surg Oncol. 2016;23:3616–22. https://doi.org/10.1245/s10434-016-5290-4.
Furrer M, Rechsteiner R, Eigenmann V, Signer C, Althaus U, Ris HB. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur J Cardiothorac Surg. 1997;12:82–7. https://doi.org/10.1016/s1010-7940(97)00105-x.
Hasegawa T, Takaki H, Kodama H, Yamanaka T, Nakatsuka A, Sato Y, et al. Three-year survival rate after radiofrequency ablation for surgically resectable colorectal lung metastases: a prospective multicenter study. Radiology. 2020;294:686–95. https://doi.org/10.1148/radiol.2020191272.
Kurilova I, Gonzalez-Aguirre A, Beets-Tan RG, Erinjeri J, Petre EN, Gonen M, et al. Microwave ablation in the management of colorectal cancer pulmonary metastases. Cardiovasc Intervent Radiol. 2018;41:1530–44. https://doi.org/10.1007/s00270-018-2000-6.
Sharma A, Baker S, Duijm M, Oomen-de Hoop E, Cornelissen R, Verhoef C, et al. Prognostic factors for local control and survival for inoperable pulmonary colorectal oligometastases treated with stereotactic body radiotherapy. Radiother Oncol. 2020;144:23–9. https://doi.org/10.1016/j.radonc.2019.10.004.
Nicosia L, Franceschini D, Perrone-Congedi F, Casamassima F, Gerardi MA, Rigo M, et al. A multicenter LArge retrospectIve daTabase on the personalization of stereotactic ABlative radiotherapy use in lung metastases from colon-rectal cancer: The LaIT-SABR study. Radiother Oncol. 2022;166:92–9. https://doi.org/10.1016/j.radonc.2021.10.023.
Karam E, Tabutin M, Mastier C, Crignis L, Peyrat P, Martin V, et al. Curative-intent treatment of pulmonary metastases from colorectal cancer: a comparison between imaging-guided thermal ablation and surgery. J Surg Oncol. 2022. https://doi.org/10.1002/jso.27108.
Nelson DB, Tayob N, Nguyen QN, Erasmus J, Mitchell KG, Hofstetter WL, et al. Local failure after stereotactic body radiation therapy or wedge resection for colorectal pulmonary metastases. J Thorac Cardiovasc Surg. 2019;158(1234–1241): e16. https://doi.org/10.1016/j.jtcvs.2019.02.133.
Widder J, Klinkenberg TJ, Ubbels JF, Wiegman EM, Groen HJ, Langendijk JA. Pulmonary oligometastases: metastasectomy or stereotactic ablative radiotherapy? Radiother Oncol. 2013;107:409–13. https://doi.org/10.1016/j.radonc.2013.05.024.
Shah SA, Haddad R, Al-Sukhni W, Kim RD, Greig PD, Grant DR, et al. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006;202:468–75. https://doi.org/10.1016/j.jamcollsurg.2005.11.008.
Wei AC, Coburn NG, Devitt KS, Serrano PE, Moulton CA, Cleary SP, et al. Survival following resection of intra- and extra-hepatic metastases from colorectal cancer: a phase II trial. Ann Surg Oncol. 2016;23:2644–51. https://doi.org/10.1245/s10434-016-5189-0.
Adam R, de Haas RJ, Wicherts DA, Vibert E, Salloum C, Azoulay D, et al. Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann Surg. 2011;253:349–59. https://doi.org/10.1097/SLA.0b013e318207bf2c.
Pulitano C, Bodingbauer M, Aldrighetti L, de Jong MC, Castillo F, Schulick RD, et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann Surg Oncol. 2011;18:1380–8. https://doi.org/10.1245/s10434-010-1459-4.
Leung U, Gonen M, Allen PJ, Kingham TP, DeMatteo RP, Jarnagin WR, et al. Colorectal cancer liver metastases and concurrent extrahepatic disease treated with resection. Ann Surg. 2017;265:158–65. https://doi.org/10.1097/SLA.0000000000001624.
Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317:2392–401. https://doi.org/10.1001/jama.2017.7105.
Chmura S, Winter KA, Robinson C, Pisansky TM, Borges V, Al-Hallaq H, et al. Evaluation of safety of stereotactic body radiotherapy for the treatment of patients with multiple metastases: findings from the NRG-BR001 phase 1 trial. JAMA Oncol. 2021;7:845–52. https://doi.org/10.1001/jamaoncol.2021.0687.
Harrow S, Palma DA, Olson R, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic Radiation for the Comprehensive Treatment of Oligometastases (SABR-COMET): extended long-term outcomes. Int J Radiat Oncol Biol Phys. 2022;114:611–6. https://doi.org/10.1016/j.ijrobp.2022.05.004.
Palma DA, Olson R, Harrow S, Correa RJM, Schneiders F, Haasbeek CJA, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4–10 oligometastatic tumors (SABR-COMET-10): study protocol for a randomized phase III trial. BMC Cancer. 2019;19:816. https://doi.org/10.1186/s12885-019-5977-6.
Ashram S, Bahig H, Barry A, Blanchette D, Celinksi A, Chung P, et al. Planning trade-offs for SABR in patients with 4 to 10 metastases: a substudy of the SABR-COMET-10 randomized trial. Int J Radiat Oncol Biol Phys. 2022. https://doi.org/10.1016/j.ijrobp.2022.05.035.
Sheikh S, Chen H, Sahgal A, Poon I, Erler D, Badellino S, et al. An analysis of a large multi-institutional database reveals important associations between treatment parameters and clinical outcomes for stereotactic body radiotherapy (SBRT) of oligometastatic colorectal cancer. Radiother Oncol. 2022;167:187–94. https://doi.org/10.1016/j.radonc.2021.12.018.
Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–18. https://doi.org/10.1056/NEJMoa2017699.
Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386:2363–76. https://doi.org/10.1056/NEJMoa2201445.
Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566–76. https://doi.org/10.1038/s41591-020-0805-8.
Newhook TE, Overman MJ, Chun YS, Dasari A, Tzeng CD, Cao HST, et al. Prospective study of perioperative circulating tumor DNA dynamics in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2022. https://doi.org/10.1097/SLA.0000000000005461.
Acknowledgements
The authors would like to thank Jennifer Huber for editorial assistance.
Funding
EDM is supported by a NIH/NCI Cancer Center Support Grant (P30 CA16058). PBR is supported by a NIH/NCI Cancer Center Support Grant (P30 CA008748) and a NIH/NCI early career development award (K08 CA255574).
Author information
Authors and Affiliations
Contributions
All authors participated in drafting and critical review of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
EDM has served as a consultant for Varian Medical Systems. KEH reports research relationships with Elekta, Merck, and BioMimetix that are unrelated to the topic of this review. PBR received research funding and serves as a consultant for EMD Serono, receives research funding from XRAD Therapeutics, is a consultant for Faeth therapeutics, and is a consultant for Natera.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miller, E.D., Hitchcock, K.E. & Romesser, P.B. Oligometastatic Colorectal Cancer: A Review of Definitions and Patient Selection for Local Therapies. J Gastrointest Canc 54, 1116–1127 (2023). https://doi.org/10.1007/s12029-022-00900-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00900-5