Abstract
Background
To analyze long-term oncological outcomes of open and percutaneous thermal ablation in the treatment of patients with colorectal liver metastases (CRLM).
Methods
This assessment from a prospective, longitudinal tumor registry included 329 patients who underwent 541 procedures for 1350 CRLM from January 2010 to February 2021. Three cohorts were formed: 2010–2013 (129 procedures [53 percutaneous]), 2014–2017 (206 procedures [121 percutaneous]) and 2018–2021 (206 procedures [135 percutaneous]). Local tumor progression-free survival (LTPFS) and overall survival (OS) data were estimated using the Kaplan–Meier method. Potential confounding factors were analyzed with uni- and multivariable Cox regression analyses.
Results
LTPFS improved significantly over time for percutaneous ablations (2-year LTPFS 37.7% vs. 69.0% vs. 86.3%, respectively, P < .0001), while LTPFS for open ablations remained reasonably stable (2-year LTPFS 87.1% [2010–2013], vs. 92.7% [2014–2017] vs. 90.2% [2018–2021], P = .12). In the latter cohort (2018–2021), the open approach was no longer superior regarding LTPFS (P = .125). No differences between the three cohorts were found regarding OS (P = .088), length of hospital stay (open approach, P = .065; percutaneous approach, P = .054), and rate and severity of complications (P = .404). The rate and severity of complications favored the percutaneous approach in all three cohorts (P = .002).
Conclusion
Over the last 10 years efficacy of percutaneous ablations has improved remarkably for the treatment of CRLM. Oncological outcomes seem to have reached results following open ablation. Given its minimal invasive character and shorter length of hospital stay, whenever feasible, percutaneous procedures may be favored over an open approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal liver metastases (CRLM) develop in up to 50% of patients with colorectal cancer, unfortunately only one-fifth of these patients are eligible for curative local treatment [1,2,3,4,5,6]. Most consider surgical resection the golden standard in upfront resectable CRLM, however, the deep-rooted mantra that surgical resection is the only curative intent treatment option for CRLM seems no longer factual [1, 2, 4, 7]. Radiofrequency (RFA) and microwave (MWA) ablation have proved themselves to result in cure in selected patients and consequently became routine treatment options for smaller-size hepatocellular carcinoma (≤ 2 cm) and unresectable small (≤ 3 cm) CRLM [1, 4, 8,9,10] .
Thermal ablation can be performed via an open, laparoscopic or percutaneous approach. Laparoscopic ablation is increasingly being performed due to its minimal invasive character compared to ablations via laparotomy, and local control rates are reported to be comparable between the two approaches. [11] However, laparoscopic ablation is technically more demanding and requires a fairly high level of expertise, which is presumably the reason that it is not yet widely embraced worldwide [4, 12, 13]. The percutaneous approach is mainly preferred in patients whose comorbid conditions preclude surgery, for centrally located tumors otherwise requiring a major resection (parenchyma-sparing), or in patients with regional or local tumor progression after prior local liver treatment [14,15,16,17,18]. This minimally invasive percutaneous approach is known for its favorable safety profile with low major complications rates (1.3%–2.4%) [14, 19, 20] .
Thermal ablation procedures have developed rapidly in terms of a potential learning curve effect, extensively upgraded device specifications, optimization of anesthetic techniques, use of image guidance tools and image fusion software platforms for volumetric assessment of the ablation zone [6, 7, 21,22,23,24,25,26]. When it comes to analyzing the efficacy and improvement of a certain treatment modality, the technique to eradicate tumors can best be elucidated by analyzing local control and time-to-local tumor progression [2, 27]. Local tumor progression (LTP) rates after thermal ablation of CRLM vary widely in the literature, ranging 7.6–22.2% for patients treated by percutaneous procedures and 2.7–9.5% for patients treated by open ablation [7, 11, 28,29,30,31,32]. Median overall survival (OS) rates after thermal ablation are reported mainly in matched cohorts or after multivariable analysis and vary from 34.3 to 53.2 months with 5- and 10-year survival rates of 20.8–60.0% and 18.0%, respectively [9, 19, 20, 33,34,35,36,37,38,39] .
As oncological outcomes of thermal liver ablation differ substantially among semi-recently published papers and evidence regarding the potential improvement over time, in terms of local control and time-to-local tumor progression, is lacking, this single-center Amsterdam Colorectal Liver Met Registry (AmCORE) based study aimed to analyze local disease control and survival outcomes following thermal ablation in patients treated for hepatic metastases from colorectal cancer over the last 10 years.
Material and Methods
Patients
Data were sourced from a prospective, longitudinal tumor registry for patients with hepatic metastases from colorectal cancer. All patients were treated at the Amsterdam UMC, location Vrije Universiteit (Amsterdam, the Netherlands), a tertiary referral institution for hepatobiliary and gastrointestinal malignancies. Approval was granted from the affiliated Institutional Review Board (reference number 2021.0121).
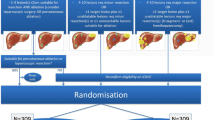
Between January 2010 and February 2021, 449 consecutive patients with liver-only metastatic colorectal carcinoma underwent open or percutaneous thermal ablation with RFA or MWA (Fig. 1). One-hundred fifteen patients were excluded for having no available follow-up data at our institute. Although higher morbidity rates have never been reported after simultaneous liver ablation and bowel resection, partial hepatectomy plus colon surgery is known to be associated with a significant increased postoperative morbidity rates [40]. To overcome potential outcome interference, 15 patients were excluded having received simultaneous bowel resection. The remaining 329 patients underwent 541 procedures for 1350 liver metastases. Preprocedural treatment planning (e.g., angle of probe insertion) was performed prior to all procedures, and for percutaneous sessions, all needles/antennae were inserted under real-time computed tomography (CT) imaging. All patients had an Eastern Cooperative Oncology Group status of ≤ 2. The diagnosis of CRLM was based on cross-sectional imaging containing CT, magnetic resonance imaging (MRI) and [18F]-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography (PET)–CT scans. Treatment planning was routinely discussed in a multidisciplinary tumor board. An open rather than a percutaneous approach was chosen in case of liver metastases needing concomitant partial hepatectomy or when a percutaneous approach was technically not feasible due to the position of the tumor (e.g., in close proximity to the stomach). Although induction systemic therapy is not standard of care within the Netherlands, three patient categories did often receive induction systemic therapy first, namely: (A) patients with locally advanced primary (rectal) cancer, (B) patients with unresectable but potentially downstagable CRLM or with difficultly resectable disease if systemic therapy is likely to reduce procedural risk, and (C) patients with early metachronous disease. Chemotherapy regimen consisted of either capecitabine or irinotecan monotherapy, capecitabine and oxaliplatin (CAPOX), capecitabine + irinotecan (CAPIRI), folinic acid + 5-fluorouracil + oxaliplatin (FOLFOX) or folinic acid + 5-fluorouracil + irinotecan (FOLFIRI). Additional monoclonal antibodies (bevacizumab or panitumumab) were added in case of potentially downstagable disease. Conformal to national guidelines, no patients received adjuvant systemic therapy. [41].
The baseline characteristics of all enrolled patients are summarized in Table 1. Of 541 procedures, 232 were performed intraoperatively and 309 under CT guidance. A total of 653 metastases were treated with RFA (481 by open approach; 172 percutaneous) and 697 metastases with MWA (327 open and 370 percutaneous). A total of 171 procedures (31.6%) were performed after induction chemotherapy. The median number of treated tumors per procedure was 2.0 (IQR 3.0) in the entire cohort. Of 232 open procedures for 808 metastases, 449 (55.6%) metastases were ablated in the same session as concurrent partial hepatectomy was performed. Median follow-up time after each ablation was 16.5 months (IQR 26.8) in the entire cohort.
Ablation Method
The vast majority of open and percutaneous ablations were performed by three interventional radiologists (BM, JV, MM) who have performed and/or supervised > 100 image-guided tumor ablations. The staff in our department has been almost stable over the last ten years. Approximately one-third of the procedures were performed by two interventional radiologists at the same time. During approximately 60% of all ablation procedures, the senior interventional radiologist (MM) was present. The procedure and other study-related details are given in supplementary materials (Appendix 1).
Efficacy Evaluation and Follow-Up Strategy
Within the first two weeks after the initial procedure, a quality control contrast-enhanced CT scan was performed when there was a potential inadequate safety margin (0–5 mm) in combination with sub-optimal tumor conspicuity and needle visibility during the procedure [6]. This allowed for an early completion ablation procedure, if indicated. Follow-up should have consisted of at least one cross-sectional imaging modality study to reliable exclude or detect LTP. Regular follow-up consisted of [18]F-FDG-PET CT scans every 3 months after the initial ablation during the first year of follow-up and roughly every 6 months thereafter, according to national guidelines [41] and the standardization paper [2]. Additional MRI was only performed in case of uncertainty whether LTP was present. Follow-up imaging was reviewed by the interventional oncology team, certified diagnostic abdominal radiologists and nuclear physicians. If loco-regional disease recurrence was found on follow-up imaging, optimal retreatment was offered based on recommendations of the multidisciplinary team, depending on the extent of the disease in the liver, hepatic function, extrahepatic metastases and general condition of the patient.
Data Collection and Statistical Analysis
For the sake of oncological outcome analyses, the entire cohort was divided into three subgroups (2010–2013, 2014–2017 and 2018–2021). Standard demographic, clinical and surveillance data were retrieved from the electronic database. Categorical variables are reported as frequencies (with or without percentage; %), whereas continuous variables are presented as median (IQR, interquartile range) or mean (± SD, standard deviation). Differences between the three subgroups in terms of baseline variables and outcomes were determined by using the Pearson Chi-square (χ2) test for categorical variables (a) and the one-way ANOVA (b) for comparison of means between the three subgroups.
Endpoint definitions were used along the consensus guidelines for the definition of time-to-event endpoints in image-guided tumor ablation by Puijk et al. [27] To study the primary endpoint, a time-to-event superiority analysis was used to analyze local tumor progression. LTP was defined as growth of tumor tissue at the initial treated tumor site [2, 27]. Patients were followed until the first recorded evidence of LTP (event) or until the last follow-up exam for those alive without LTP. Local tumor progression-free survival (LTPFS) curves, per patient and per tumor, were estimated using the Kaplan–Meier method and compared between subgroups using the log-rank test. Death without LTP was considered a competing risk. LTPFS over time was analyzed by allocating patients into one of three historical cohorts (2010–2013; 2014–2017 and 2018–2021). Baseline variables with P-values < .05 were entered in the univariable analysis. Uni- and multivariable analyses for LTPFS were performed by using the Cox proportional hazard regression model in the entire cohort. Variables with P < .05 in the univariable analysis were included in the final multivariable model. Hazard ratios (HR) and 95 percent confidence intervals (95% CI) were calculated. Using backward selection procedure, results of step-by-step removed variables were reported. Results are from last step before removal. Secondary endpoints were overall survival (OS) and safety. OS probability was estimated using the Kaplan–Meier method (time from the first ablation until the date of death or to the last follow-up visit or exam) for the entire cohort. Death during the index hospitalization or within 30 days after treatment was considered perioperative mortality. Safety in terms of complications was evaluated and reported using the standardized Common Terminology Criteria for Adverse Events (CTCAE) grading system, version 4.0 and 5.0. [2, 27, 42].
Statistical analyses were performed in consultation with an independent statistician (BLW) using SPSS® software, version 24.0 (IBM®, Armonk, New York, USA) [43] and the R software package, version 3.6.3 (R Foundation, Vienna, Austria) [44]. Statistical significance was established for P < .05. All results were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational study data. [45].
Results
Technical Success and Local Tumor Progression
A total of 329 patients (mean age, 65.3 years ± 10.8; 222 men) met the inclusion criteria (Fig. 1 and Table 1). Incomplete ablation rate was 1.0% (14/1350), identified on early follow-up imaging and retreated within ten weeks following the initial ablation. The cumulative LTP rate after 6 months, 1, 2 and 3 years follow-up was 7.4% (100/1350), 11.6% (156/1350), 13.6% (183/1350) and 13.9% (186/1350), respectively, in the entire cohort demonstrated in Table 2 and illustrated as Kaplan–Meier estimates of LTPFS in Fig. 2. For small-size metastases only (≤ 3 cm) (n = 1125), the cumulative LTP rate was 10.7% (120/1125) during a median follow-up duration of 17.5 months (IQR 27.1).
Kaplan–Meier survival curves indicating local tumor progression-free survival (LTPFS) per treated tumor (A) and per patient (B) after all thermal ablation sessions. Numbers at risk correspond to the amount of tumors and number of patients respectively. Death without local tumor progression (LTP) is censored (competing risk)
Multivariable analysis revealed four factors associated with an inferior LTPFS (Table 3): no induction chemotherapy (HR 0.480, P < .001), percutaneous approach (HR 4.265, P < .001), larger size of metastasis (HR 1.932 for intermediate size [31-50 mm] and HR 4.783 for large size [> 50 mm], P < .001). Adjusted HR of ablations performed between 2014–2017 compared to 2010–2013 was 0.437 (95% CI 0.301–0.636) and 2018–2021 compared to 2010–2013 was 0.244 (95% CI 0.142–0.419) (P < .001).
LTPFS per time frame is demonstrated in Fig. 3 (P < .0001). A per approach sub-analysis revealed LTP rates of 7.9% (64/808) for liver metastases treated with open ablation and 22.5% (122/542) for percutaneously ablated tumors, as shown in Fig. 4. The two-year LTPFS rate improved from 37.7% (2010–2013), to 69.0% (2014–2017) to 86.3% (2018–2021) (P < .0001) for patients treated with percutaneous ablation, while no temporal difference was found in LTPFS for patients treated with open ablation (87.1% vs 92.7% vs 90.2%, respectively; P = .12) (Fig. 4c-f; P-values in chart). Improvement in LTPFS was most remarkable after percutaneous ablation—as such, sub-analysis of all percutaneous procedures was performed to evaluate potential influencing factors. Procedure- and tumor-related characteristics of all percutaneous ablations are listed in Appendix 2a and 2b. Multivariable analysis revealed two factors associated with a superior LTPFS: Anesthetic management (HR 0.296 for propofol sedation and HR 0.978 for general anesthesia compared to midazolam + fentanyl sedation, P < .001). Adjusted HR of percutaneous ablations performed between 2014 and 2017 compared to 2010–2013 was 0.495 (95% CI (0.289–0.847) and 2018–2021 compared to 2010–2013 was 0.221 (95% CI 0.107–0.459) (P < .001).
Kaplan–Meier survival curves indicating the local tumor progression-free survival (LTPFS) per time frame. Analysis per treated tumor (A) and per patient (B). Time frames: 2010–2013; 2014–2017 and 2018–2021. Numbers at risk correspond to the amount of tumors and number of patients respectively. Overall comparison log-rank (Mantel–Cox) test is reported per graph. Death without local tumor progression (LTP) is censored (competing risk).
Kaplan–Meier survival curves indicating local tumor progression-free survival (LTPFS) per time frame and approach. A, B Analysis of open and percutaneous thermal ablation per treated tumor and per patient respectively, C and D patients treated with open ablation, analysis per treated tumor and per patient respectively, E and F patients treated with percutaneous ablation, analysis per treated tumor and per patient respectively. Numbers at risk correspond to either the amount of tumors or the number of patients. Overall comparison log-rank (Mantel–Cox) test is reported per graph. Death without local tumor progression (LTP) is censored (competing risk)
Complications and Length of Hospital Stay
Grade 1–5 complication rate in the entire cohort was 20.5% (111/541 procedures; Table 2). The severity of complications did not change over time (P = .404). The rate and severity of complications favored the percutaneous approach in all three cohorts (2010–2013, P = .069; 2014–2017, P = .129; 2018–2021, P = .020). Sub-analysis of procedures were thermal ablation was used solely (in other words without simultaneous resection or irreversible electroporation in case of open procedures), revealed no difference in complication rate between the three time frames (P = .406).
Overall procedure-related mortality was 1.5% (5/329) in the entire cohort. One patient deceased 7 days after combined liver resection and ablation due to massive pulmonary embolism (30-day mortality 0.4%; n = 1/329). Five others died from postoperative complications between 30 and 90 days: one due to massive portal thrombosis and multi-organ failure 5 weeks after combined percutaneous ablation and irreversible electroporation, and three due to abdominal abscesses and cardiopulmonary failure 8–9 weeks after combined liver resection and open ablation.
For open ablations, the mean length of hospital stay did not significantly differ between the three time frames (mean 6.9 days [SD 5.9]; P = .065). Mean hospitalization after percutaneous procedures was 1.4 days (SD 2.6) with no differences between the three cohorts (P = .054).
Overall Survival
A total of 99 patients (30.1%) deceased during follow-up (Table 2). Of them, 93 died from disease progression. Survival probability after the first ablative treatment was 92.0%, 78.8%, 45.9% and 26.8% at 1, 3, 5 and 10 years, respectively (Fig. 5). For the entire cohort, the median OS after the first ablation procedure was 54.2 months; 52.0 months in the 2010–2013 cohort and 66.6 months in the 2014–217 cohort. The median OS for the latter cohort was not met. The median OS did not significantly improve over the last decade (P = .088), nor differed for patients treated by open or percutaneous ablation (P = .888).
Discussion
Over the past decades, thermal ablation has become the standard treatment option to eradicate small unresectable CRLM (≤ 3 cm) and a fair alternative for deep-seated resectable CRLM that would otherwise require major hepatectomy [1, 2, 4]. Though advances in energy delivery in methods for precise probe placement and in ablation confirmation techniques have, often prematurely, been introduced as alleged improvements, our results underwrite technological progresses made over time. The improvement over time, in terms of LTPFS, especially for patients being treated with CT-guided percutaneous ablations, was the most remarkable finding in our study. OS did not significantly improve over the last 10 years. Whether this reflects an absent correlation between survival and local treatment failure, especially given the relative ease to repeat ablations, or the gradual acceptance to offer curative intent ablations to more complex cases with higher disease burden, remains unknown.
Results of this study compare well with OS and LTPFS data published in other recent series regarding thermal ablation of CRLM [1, 14, 35,36,37,38,39, 46, 47]. We have reached the point where the local tumor progression rate after percutaneous ablation has approached results following open ablation as well as following partial hepatectomy, as the most recent surgical series report R1/R2 rates varying from 12 to 46% [48,49,50,51,52]. Outcomes of this current cohort study are again underlining the necessity to conduct a randomized controlled trial comparing standard partial hepatectomy to its less invasive competitor thermal ablation for smaller-size resectable CRLM (≤ 3 cm). Although the phase III randomized LAVA trial (ISRCTN52040363) attempted to randomize high surgical risk CRLM patients to surgery or thermal ablation, recruitment feasibility was not established during the pilot stage, and therefore, the trial closed early without having gathered data regarding the primary endpoint two-year disease-free survival [53]. The interim results of the COLLISION trial (NCT03088150), presented at CIRSE 2021 and ECIO 2022, confirm thermal ablations’ superior safety profile, shorter hospital stay, equal to superior local control and similar OS compared to partial hepatectomy; the final results are eagerly awaited [54, 55]. Though a recent comparative analysis favored thermal ablation with regard to OS, LTPFS and eventual local control for small-size (≤ 3 cm), stereotactic body radiation therapy (SBRT) does challenge thermal ablation for intermediate-size (3-5 cm) CRLMs; the ongoing COLLISION-XL trial (NCT04081168) will hopefully provide clarity. [56].
Although speculative, the improvement over time, in terms of LTPFS, for patients being treated with percutaneous ablation should probably be contributed to (A) gained experience and (B) technological advancements made during the last decades. A multitude of minor improvements with regard to energy delivery spectrum, antenna and generator design (e.g., Thermosphere™ technology, multiple antennae systems or stereotactic navigation), anesthesia and breath-hold techniques, real-time image guidance (e.g., administration of intra-arterial contrast via an hepatic artery catheter) and the use of rigid and non-rigid image fusion and registration platforms allowing intraprocedural completion ablations seem to have led to this major quality improvement [6, 7, 22, 24,25,26, 31, 57,58,59,60,61].
Some limitations need to be addressed. The median follow-up period in the 2018–2021 cohort was sufficient (11.5 months), but inevitably lower compared to the earlier cohorts. This may have led to the situation where some patients in the latest cohort are still susceptible to developing LTP (immortality time bias), though this only applies to a small amount of tumors; as historically seen, the vast majority of LTPs are detected within the first 3–9 months following local treatment and a clear LTPFS plateau is reached after roughly 18 months follow-up (Fig. 2a) [9]. Reported study data were analyzed from prospectively kept records, and potential confounders were excluded by uni- and multivariable analyses, which does not fully guarantee that residual confounding has been eliminated. The fact that periprocedural chemotherapy regimens and follow-up imaging protocols did not change over time decreases the likelihood for residual bias. The lack of a comparison between laparoscopic and open ablated tumors could be a potential limitation as in certain cases the laparoscopic approach might be superior to the open approach in terms of safety and length of hospital stay. Due to technological advancements in energy delivery and reduced procedure time, MWA was gradually favored over RFA, even though previously published data showed no significant difference in terms of local disease control [6, 60, 61]. Nonetheless, the ablation modality need to be addressed as potential confounder. In addition, the specific ablation devices used in this study may render the comparative results as they do not necessarily represent all current day ablation systems. Although mutant RAS and BRAF status are known to be associated with LTP [47, 62], these tumor characteristics were not routinely measured over the last decade, resulting in high rates of missing data. Furthermore, it should be noted that the national guideline recommendations not routinely offer neo-adjuvant or adjuvant chemotherapy for locally treatable disease, what differs from several other countries and regions, and hence, it may be challenging to compare our results with series where patients were routinely offered (neo-)adjuvant systemic therapy [41]. However, the national guideline recommendations did not change over time and were actually re-established following the recent publication of two clinical trials of which one showed no difference in OS for perioperative chemotherapy (EORTC 40983) [63] and one showed an inferior OS for adding adjuvant chemotherapy (JCOG 0603) [64] .
In conclusion, the efficacy of percutaneous ablations for CRLM in terms of local tumor progression-free survival has improved remarkably over the last 10 years and seems to have approached oncological outcomes following open ablations. Over the last decade, no differences were found regarding length of hospital stay, rate and severity of complications, and overall survival. Given its minimal invasive character and shorter length of hospital stay, whenever feasible, percutaneous procedures may be favored over an open approach.
References
Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41:1189–204.
Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273:241–60.
Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33:11–7.
Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontiers meeting 2013. Eur Radiol. 2015;25:3438–54.
Bala MM, Riemsma RP, Wolff R, et al. Microwave coagulation for liver metastases. Cochrane Database Syst Rev. 2013;1:010163.
Puijk RS, Ruarus AH, Scheffer HJ, et al. Percutaneous liver tumour ablation: image guidance, endpoint assessment, and quality control. Can Assoc Radiol J. 2018;69:51–62.
de Baere T, Tselikas L, Yevich S, et al. The role of image-guided therapy in the management of colorectal cancer metastatic disease. Eur J Cancer. 2017;75:231–42.
Salhab M, Canelo R. An overview of evidence-based management of hepatocellular carcinoma: a meta-analysis. J Cancer Res Ther. 2011;7:463–75.
Nielsen K, van Tilborg AA, Meijerink MR, et al. Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J Surg. 2013;37:1340–7.
Vasiniotis Kamarinos N, Kaye EA, Sofocleous CT. Image-guided thermal ablation for colorectal liver metastases. Tech Vasc Interv Radiol. 2020;23: 100672.
Giglio MC, Logghe B, Garofalo E, et al. Laparoscopic versus open thermal ablation of colorectal liver metastases: a propensity score-based analysis of local control of the ablated tumors. Ann Surg Oncol. 2020;27:2730.
Santambrogio R, Bianchi P, Pasta A, et al. Ultrasound-guided interventional procedures of the liver during laparoscopy: technical considerations. Surg Endosc. 2002;16:349–54.
Takahashi H, Berber E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr. 2020;9:49–58.
Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology. 2016;278:601–11.
Elias D, De Baere T, Smayra T, et al. Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg. 2002;89:752–6.
Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22:755–61.
Livraghi T, Solbiati L, Meloni F, et al. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach.” Cancer. 2003;97:3027–35.
Nieuwenhuizen S, Puijk RS, van den Bemd B, et al. Resectability and ablatability criteria for the treatment of liver only colorectal metastases: multidisciplinary consensus document from the COLLISION Trial Group. Cancers (Basel). 2020;12:1779.
Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–68.
Shi Y, Wang Z, Chi J, et al. Long-term results of percutaneous microwave ablation for colorectal liver metastases. HPB (Oxford). 2021;23:37–45.
Puijk RS, Des Plantes VZ, Nieuwenhuizen S, et al. Propofol compared to midazolam sedation and to general anesthesia for percutaneous microwave ablation in patients with hepatic malignancies: a single-center comparative analysis of three historical cohorts. Cardiovasc Intervent Radiol. 2019;42:1579–2068.
Puijk RS, Nieuwenhuizen S, van den Bemd BAT, et al. Transcatheter CT hepatic arteriography compared with conventional CT fluoroscopy guidance in percutaneous thermal ablation to treat colorectal liver metastases: a single-center comparative analysis of 2 historical cohorts. J Vasc Interv Radiol. 2020;31:1772–83.
Solbiati M, Muglia R, Goldberg SN, et al. A novel software platform for volumetric assessment of ablation completeness. Int J Hyperthermia. 2019;36:337–43.
Kaye EA, Cornelis FH, Petre EN, et al. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol. 2019;29:2698–705.
Laimer G, Schullian P, Bale R. Stereotactic thermal ablation of liver tumors: 3D planning, multiple needle approach, and intraprocedural image fusion are the key to success-a narrative review. Biology (Basel). 2021;10:644.
Laimer G, Jaschke N, Schullian P, et al. Volumetric assessment of the periablational safety margin after thermal ablation of colorectal liver metastases. Eur Radiol. 2021;31:6489–99.
Puijk RS, Ahmed M, Adam A, et al. Consensus guidelines for the definition of time-to-event end points in image-guided tumor ablation: results of the SIO and DATECAN initiative. Radiology. 2021;301:533.
Vietti Violi N, Duran R, Demartines N, et al. Local recurrence rate in patients with colorectal cancer liver metastasis after wedge resection or percutaneous radiofrequency ablation. Int J Hyperthermia. 2018;34:1020–8.
Yu J, Liang P, Yu XL, et al. Local tumour progression after ultrasound-guided microwave ablation of liver malignancies: risk factors analysis of 2529 tumours. Eur Radiol. 2015;25:1119–26.
Wong J, Lee KF, Yu SC, et al. Percutaneous radiofrequency ablation versus surgical radiofrequency ablation for malignant liver tumours: the long-term results. HPB (Oxford). 2013;15:595–601.
Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29:268–75.
Elias D, Baton O, Sideris L, et al. Local recurrences after intraoperative radiofrequency ablation of liver metastases: a comparative study with anatomic and wedge resections. Ann Surg Oncol. 2004;11:500–5.
Hamada A, Yamakado K, Nakatsuka A, et al. Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol. 2012;30:567–74.
Tsitskari M, Filippiadis D, Zavridis P, et al. Efficacy and safety of percutaneous computed tomography-guided microwave ablation for colorectal cancer, oligometastatic liver-only disease: a single center’s experience. Ann Gastroenterol. 2021;34:61–7.
Faitot F, Faron M, Adam R, et al. Two-stage hepatectomy versus 1-stage resection combined with radiofrequency for bilobar colorectal metastases: a case-matched analysis of surgical and oncological outcomes. Ann Surg. 2014;260:822–7 (discussion 827-828).
Tinguely P, Dal G, Bottai M, et al. Microwave ablation versus resection for colorectal cancer liver metastases - a propensity score analysis from a population-based nationwide registry. Eur J Surg Oncol. 2020;46:476–85.
Hof J, Wertenbroek MW, Peeters PM, et al. Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg. 2016;103:1055–62.
Imai K, Allard MA, Castro Benitez C, et al. Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br J Surg. 2017;104:570–9.
Karanicolas PJ, Jarnagin WR, Gonen M, et al. Long-term outcomes following tumor ablation for treatment of bilateral colorectal liver metastases. JAMA Surg. 2013;148:597–601.
Snyder RA, Hao S, Irish W, et al. Thirty-day morbidity after simultaneous resection of colorectal cancer and colorectal liver metastasis: American College of Surgeons NSQIP Analysis. J Am Coll Surg. 2020;230:617–27.
Comprehensive Cancer Organisation the Netherlands (I.K.N.L.). National evidence-based guideline. Colorectaalcarcinoom. https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/startpagina_-_crc.html, Nov 2019. Accessed November 10, 2021
Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0 and 5.0 [Internet]. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm, Accessed November 10, 2021
IBM Corp. Released 2013. IBM® SPSS® Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.
(2019) RCT: R: A language and environment for statistical computing. R for Windows version 4.0.3. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
STROBE Statement - Checklist of items that should be included in reports of cohort studies. Version 4. Oct/Nov 2007. ISPM - University of Bern 2009. www.strobe-statement.org. Accessed 10 Nov 2021.
Eltawil KM, Boame N, Mimeault R, et al. Patterns of recurrence following selective intraoperative radiofrequency ablation as an adjunct to hepatic resection for colorectal liver metastases. J Surg Oncol. 2014;110:734–8.
Calandri M, Yamashita S, Gazzera C, et al. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018;28:2727–34.
Schiesser M, Chen JW, Maddern GJ, et al. Perioperative morbidity affects long-term survival in patients following liver resection for colorectal metastases. J Gastrointest Surg. 2008;12:1054–60.
Are C, Gonen M, Zazzali K, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300.
Hamady ZZ, Lodge JP, Welsh FK, et al. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg. 2014;259:543–8.
de Haas RJ, Wicherts DA, Flores E, et al. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–37.
Sadot E, Koerkamp BG, Leal JN, et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg. 2015;262:476–85.
Davidson B, Gurusamy K, Corrigan N, et al. Liver resection surgery compared with thermal ablation in high surgical risk patients with colorectal liver metastases: the LAVA international RCT. Health Technol Assess. 2020;24(21):1–38.
Puijk RS, Ruarus AH, Vroomen L, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18:821.
Meijerink MR, Puijk RS, van den Tol MP, Dijkstra M, et al. COLLISION trial interim results: surgery vs. MWA. https://library.cirse.org/speakers/53812. Presented at the CIRSE 2021 Summit, Virtual, Sept 25–29, 2021
Nieuwenhuizen S, Dijkstra M, Puijk RS, et al. Thermal ablation versus stereotactic ablative body radiotherapy to treat unresectable colorectal liver metastases: a comparative analysis from the prospective Amsterdam CORE Registry. Cancers. 2021;13:1.
Laimer G, Schullian P, Jaschke N, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol. 2020;30:2463.
Wright AS, Lee FT Jr, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10:275–83.
Puijk RS, des Plantes VZ, Nieuwenhuizen S, et al. Propofol compared to midazolam sedation and to general anesthesia for percutaneous microwave ablation in patients with hepatic malignancies: a single-center comparative analysis of three historical cohorts. Cardiovasc Intervent Radiol. 2019;42:1597–608.
Alonzo M, Bos A, Bennett S, et al. The emprint ablation system with thermosphere technology: one of the newer next-generation microwave ablation technologies. Semin Intervent Radiol. 2015;32:335–8.
Radosevic A, Quesada R, Serlavos C, et al. Microwave versus radiofrequency ablation for the treatment of liver malignancies: a randomized controlled phase 2 trial. Sci Rep. 2022;12:316.
Odisio BC, Yamashita S, Huang SY, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017;104:760–8.
Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–15.
Kanemitsu Y, Kato T, Shimizu Y, et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol. 2009;39:406–9.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
This study has obtained IRB approval from the Amsterdam UMC, location Vrije Universiteit (reference number 2021.0121) and the need for informed consent was waived.
Consent for Publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Puijk, R.S., Dijkstra, M., van den Bemd, B.A.T. et al. Improved Outcomes of Thermal Ablation for Colorectal Liver Metastases: A 10-Year Analysis from the Prospective Amsterdam CORE Registry (AmCORE). Cardiovasc Intervent Radiol 45, 1074–1089 (2022). https://doi.org/10.1007/s00270-022-03152-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03152-9