Abstract
Within the tumor microenvironment, the fight between the immune system and cancer influences tumor transformation. Metastasis formation is an important stage in the progression of cancer. This process is aided by cellular detachment and resistance to anoikis, which are achieved by altering intercellular signaling. Autophagy, specifically pro-survival autophagy, aids cancer cells in developing treatment resistance. Numerous studies have shown that autophagy promotes tumor growth and resistance to anoikis. To regulate protective autophagy, cancer-related genes phosphorylate both pro- and anti-apoptotic proteins. Apoptosis, a type of controlled cell death, eliminates damaged or unwanted cells. Anoikis is a type of programmed cell death in which cells lose contact with the extracellular matrix. The dysregulation of these cellular pathways promotes tumor growth and spread. Apoptosis, anoikis, and autophagy interact meticulously and differently depending on the cellular circumstances. For instance, autophagy can protect cancer cells from apoptosis by removing cellular components that are damaged and might otherwise trigger apoptotic pathways. Similarly, anoikis dysregulation can trigger autophagy by causing cellular harm and metabolic stress. In order to prevent or treat metastatic disease, specifically, targeting these cellular mechanisms may present a promising prospect for cancer therapy. This review discourses the state of our understanding of the molecular and cellular mechanisms underlying tumor transformation and the establishment of metastatic tumors. To enhance the prognosis for cancer, we highlight and discuss potential therapeutic approaches that target these processes and genes involved in them.




Similar content being viewed by others
References
Miller, K. D. et al. (2019). Cancer treatment and survivorship statistics. CA: A Cancer Journal for Clinicians, 69(5 Sep), 363–385. https://doi.org/10.3322/caac.21565.
Arneth, B. (2020) Tumor microenvironment, Medicina, 56, 1 Jan., https://doi.org/10.3390/MEDICINA56010015.
Spill, F., Reynolds, D. S., Kamm, R. D., & Zaman, M. H. (2016). Impact of the physical microenvironment on tumor progression and metastasis. Current Opinion in Biotechnology, 40(Aug), 41–48. https://doi.org/10.1016/J.COPBIO.2016.02.007.
Del Prete, A., Schioppa, T., Tiberio, L., Stabile, H., & Sozzani, S. (2017). Leukocyte trafficking in tumor microenvironment. Current Opinion in Pharmacology, 35(Aug), 40–47. https://doi.org/10.1016/J.COPH.2017.05.004.
Baghban, R., et al. (2020). Tumor microenvironment complexity and therapeutic implications at a glance. Cell Communication and Signaling, 18(1 Dec), 59 https://doi.org/10.1186/s12964-020-0530-4.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013.
Denk, D., & Greten, F. R. (2022). Inflammation: the incubator of the tumor microenvironment. Trends in Cancer, 8(11 Nov), 901–914. https://doi.org/10.1016/j.trecan.2022.07.002.
Jahanban-Esfahlan, R., Seidi, K., Manjili, M. H., Jahanban-Esfahlan, A., Javaheri, T., & Zare, P. (2019). Tumor cell dormancy: threat or opportunity in the fight against cancer. Cancers (Basel), 11(8 Aug), 1207 https://doi.org/10.3390/cancers11081207.
Bayne, L. J., et al. (2012). Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell, 21(6), 822–835. https://doi.org/10.1016/j.ccr.2012.04.025.
Ferraresi, A., et al. (2020) How autophagy shapes the tumor microenvironment in ovarian cancer, Frontiers in Oncology, 10, Dec., https://doi.org/10.3389/fonc.2020.599915.
Safa, A. R. (2022). Drug and apoptosis resistance in cancer stem cells (CSCs): a puzzle with many pieces. Cancer Drug Resistance, 5(4), 850–72. https://doi.org/10.20517/cdr.2022.20.
Nepali, P. R., & Kyprianou, N. (2023) Anoikis in phenotypic reprogramming of the prostate tumor microenvironment, Frontiers in Endocrinology (Lausanne)., 14, Apr., https://doi.org/10.3389/fendo.2023.1160267.
Li, X., He, S., & Ma, B. (2020). Autophagy and autophagy-related proteins in cancer. Molecular Cancer, 19(1 Dec), 12 https://doi.org/10.1186/s12943-020-1138-4.
Poillet-Perez, L., Sarry, J.-E., & Joffre, C. (2021). Autophagy is a major metabolic regulator involved in cancer therapy resistance. Cell Reports, 36(7), 109528. https://doi.org/10.1016/j.celrep.2021.109528.
Yin, X., Xin, H., Mao, S., Wu, G., & Guo, L. (2019) The role of autophagy in sepsis: protection and injury to organs, Frontiers in Physiology, 10, Aug., https://doi.org/10.3389/fphys.2019.01071.
White, E., & DiPaola, R. S. (2009). The double-edged sword of autophagy modulation in cancer. Clinical Cancer Research, 15(17 Sep), 5308–5316. https://doi.org/10.1158/1078-0432.CCR-07-5023.
Liu, M., et al. (2018). Cytoplasmic liver kinase B1 promotes the growth of human lung adenocarcinoma by enhancing autophagy. Cancer Science, 109(10 Oct), 3055–3067. https://doi.org/10.1111/cas.13746.
Yun, C. W., Lee, S. H. (2018) The roles of autophagy in cancer, International Journal of Molecular Sciences, 19, 11, Nov. MDPI AG. https://doi.org/10.3390/ijms19113466.
Luo, T., et al. (2016). PSMD10/gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression. Autophagy, 12(8 Aug), 1355–1371. https://doi.org/10.1080/15548627.2015.1034405.
Walczak, M., & Martens, S. (2013). Dissecting the role of the Atg12–Atg5-Atg16 complex during autophagosome formation. Autophagy, 9(3 Mar), 424–425. https://doi.org/10.4161/auto.22931.
Badadani, M. (2012) Autophagy mechanism, regulation, functions, and disorders, International Scholarly Research Network ISRN Cell Biology, 2012, 11, https://doi.org/10.5402/2012/927064.
Lee, Y.-K., & Lee, J.-A. (2016). Role of the mammalian ATG8/LC3 family in autophagy: differential and compensatory roles in the spatiotemporal regulation of autophagy. BMB Reports, 49(8 Aug), 424–430. https://doi.org/10.5483/BMBRep.2016.49.8.081.
Chen, D., Fan, W., Lu, Y., Ding, X., Chen, S., & Zhong, Q. (2012). A mammalian autophagosome maturation mechanism mediated by TECPR1 and the Atg12-Atg5 conjugate. Molecular Cell, 45(5), 629–641. https://doi.org/10.1016/j.molcel.2011.12.036.
Rouschop, K. M. A., et al. (2010). The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. Journal of Clinical Investigation, 120(1 Jan), 127–141. https://doi.org/10.1172/JCI40027.
Chen, Q., Kang, J., & Fu, C. (2018). The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduction and Targeted Therapy, 3(1 Jul), 18 https://doi.org/10.1038/s41392-018-0018-5.
Ayla, S., & Karahüseyinoğlu, S. (2019). Cancer stem cells, their microenvironment and anoikis. Critical Reviews in Oncogenesis, 24(1), 27–34. https://doi.org/10.1615/CritRevOncog.2018029433.
Sattari Fard, F., Jalilzadeh, N., Mehdizadeh, A., Sajjadian, F., & Velaei, K. (2023). Understanding and targeting anoikis in metastasis for cancer therapies. Cell Biology International, 47(4 Apr), 683–698. https://doi.org/10.1002/cbin.11970.
Corkery, D. P., et al. (2018). Loss of PRP4K drives anoikis resistance in part by dysregulation of epidermal growth factor receptor endosomal trafficking. Oncogene, 37(2 Jan), 174–184. https://doi.org/10.1038/onc.2017.318.
Schwartz, M. A. (2010). Remembrance of dead cells past: discovering that the extracellular matrix is a cell survival factor. Molecular Biology of the Cell, 21(4 Feb), 499–500. https://doi.org/10.1091/mbc.E09-07-0602. American Society for Cell Biology.
Kim, Y. N., Koo, K. H., Sung, J. Y., Yun, U. J., & Kim, H. (2012) Anoikis resistance: an essential prerequisite for tumor metastasis, International Journal of Cell Biology. https://doi.org/10.1155/2012/306879.
Barriere, G., Fici, P., Gallerani, G., Fabbri, F., & Rigaud, M. (2015) Epithelial Mesenchymal Transition: a double‐edged sword, Clinical and Translational Medicine, 4, 1, Dec. https://doi.org/10.1186/s40169-015-0055-4.
Heerboth, S. et al., (2015) EMT and tumor metastasis, Clinical and Translational Medicine, 4, 1, Dec. https://doi.org/10.1186/s40169-015-0048-3.
RUSSO, M. A., et al. (2013). A small-molecule RGD-integrin antagonist inhibits cell adhesion, cell migration and induces anoikis in glioblastoma cells. International Journal of Oncology, 42(1 Jan), 83–92. https://doi.org/10.3892/ijo.2012.1708.
Zhong, X., & Rescorla, F. J. (2012). Cell surface adhesion molecules and adhesion-initiated signaling: Understanding of anoikis resistance mechanisms and therapeutic opportunities. Cell Signaling, 24(2 Feb), 393–401. https://doi.org/10.1016/j.cellsig.2011.10.005.
Wang, J., et al. (2022). Anoikis-associated lung cancer metastasis: mechanisms and therapies. Cancers (Basel), 14(19 Sep), 4791 https://doi.org/10.3390/cancers14194791.
Kim, J. Y., et al. (2017). Disulfiram induces anoikis and suppresses lung colonization in triple-negative breast cancer via calpain activation. Cancer Letters, 386(Feb), 151–160. https://doi.org/10.1016/j.canlet.2016.11.022.
Inge, L. J., et al. (2011). Soluble E-cadherin promotes cell survival by activating epidermal growth factor receptor. Experimental Cell Research, 317(6 Apr), 838–848. https://doi.org/10.1016/j.yexcr.2010.12.025.
Zoppi, N., Chiarelli, N., Ritelli, M., & Colombi, M. (2018). Multifaced roles of the αvβ3 integrin in Ehlers–Danlos and arterial tortuosity syndromes’ dermal fibroblasts. International Journal of Molecular Science, 19(4 Mar), 982. https://doi.org/10.3390/ijms19040982.
Stanislovas, J., & Kermorgant, S.(2022) c-Met-integrin cooperation: Mechanisms, tumorigenic effects, and therapeutic relevance, Frontiers in Cell and Developmental Biology, 10, Oct., https://doi.org/10.3389/fcell.2022.994528.
Boppart, M. D., & Mahmassani, Z. S. (2019). Integrin signaling: linking mechanical stimulation to skeletal muscle hypertrophy. American Journal of Physiology, 317(4 Oct), C629–C641. https://doi.org/10.1152/ajpcell.00009.2019.
Rocchetti, M. T., Bellanti, F., Zadorozhna, M., Fiocco, D., & Mangieri, D. (2023). Multi-faceted role of luteolin in cancer metastasis: EMT, angiogenesis, ECM degradation and apoptosis. International Journal of Molecular Science, 24(10 May), 8824 https://doi.org/10.3390/ijms24108824.
Deng, Z., Wang, H., Liu, J., Deng, Y., & Zhang, N. (2021). Comprehensive understanding of anchorage-independent survival and its implication in cancer metastasis. Cell Death & Disease, 12(7 Jun), 629 https://doi.org/10.1038/s41419-021-03890-7.
Khan, S. U., Fatima, K., & Malik, F. (2022). Understanding the cell survival mechanism of anoikis-resistant cancer cells during different steps of metastasis. Clinical & Experimental Metastasis, 39(5 Oct), 715–726. https://doi.org/10.1007/s10585-022-10172-9.
Wong, T. S., Gao, W., & Chan, J. Y. W. (2014) Interactions between E-cadherin and microrna deregulation in head and neck cancers: the potential interplay, BioMed Research International, Hindawi Publishing Corporation, 2014. https://doi.org/10.1155/2014/126038.
Hayashida, T., Jinno, H., Kitagawa, Y., & Kitajima, M. (2011) Cooperation of cancer stem cell properties and epithelial-mesenchymal transition in the establishment of breast cancer metastasis, Journal of Oncology. https://doi.org/10.1155/2011/591427.
Babaei, G., Aziz, S. G.-G., & Jaghi, N. Z. Z. (2021). EMT, cancer stem cells and autophagy; the three main axes of metastasis. Biomedicine & Pharmacotherapy, 133(Jan), 110909 https://doi.org/10.1016/j.biopha.2020.110909.
Strauss, S. J., Ng, T., Mendoza‐Naranjo, A., Whelan, J., & Sorensen, P. H. B. (2010). Understanding micrometastatic disease and anoikis resistance in ewing family of tumors and osteosarcoma. Oncologist, 15(6 Jun), 627–635. https://doi.org/10.1634/theoncologist.2010-0093.
Taddei, M., Giannoni, E., Fiaschi, T., & Chiarugi, P. (2012). Anoikis: an emerging hallmark in health and diseases. The Journal of Pathology, 226(2 Jan), 380–393. https://doi.org/10.1002/path.3000.
Chaurasia, M., Gupta, S., Das, A., Dwarakanath, B. S., Simonsen, A., & Sharma, K. (2019). Radiation induces EIF2AK3/PERK and ERN1/IRE1 mediated pro-survival autophagy. Autophagy, 15(8 Aug), 1391–1406. https://doi.org/10.1080/15548627.2019.1582973.
Sakamoto, S., & Kyprianou, N. (2010). Targeting anoikis resistance in prostate cancer metastasis. Molecular Aspects of Medicine, 31(2 Apr), 205–214. https://doi.org/10.1016/j.mam.2010.02.001. NIH Public Access.
Joshi, V., Upadhyay, A., Prajapati, V. K., & Mishra, A. (2020). How autophagy can restore proteostasis defects in multiple diseases? Medical Research Reviews, 40(4 Jul), 1385–1439. https://doi.org/10.1002/med.21662.
Soto-Burgos, J., Zhuang, X., Jiang, L., & Bassham, D. C. (2018). Dynamics of autophagosome formation. Plant Physiology, 176(1 Jan), 219–229. https://doi.org/10.1104/pp.17.01236.
Nguyen, J. A., & Yates, R. M. (2021) Better together: current insights into phagosome-lysosome fusion. Frontiers in Immunology, 12, Feb., https://doi.org/10.3389/fimmu.2021.636078.
Hollenstein, D. M., & Kraft, C. (2020). Autophagosomes are formed at a distinct cellular structure. Current Opinion in Cell Biology, 65(Aug), 50–57. https://doi.org/10.1016/j.ceb.2020.02.012.
Wang, Y., et al. (2023). Construction and validation of a prognostic model based on autophagy-related genes for hepatocellular carcinoma in the Asian population. BMC Genomics, 24(1 Jun), 357 https://doi.org/10.1186/s12864-023-09367-5.
Shi, X., Yokom, A. L., Wang, C., Young, L. N., Youle, R. J., & Hurley, J. H. (2020) ULK complex organization in autophagy by a C-shaped FIP200 N-terminal domain dimer, Journal of Cell Biology, 219, 7 Jul., https://doi.org/10.1083/jcb.201911047.
Zhou, Y., Manghwar, H., Hu, W., & Liu, F. (2022). Degradation mechanism of autophagy-related proteins and research progress. International Journal of Molecular Science, 23(13 Jun), 7301 https://doi.org/10.3390/ijms23137301.
Kaur, S., & Changotra, H. (2020). The beclin 1 interactome: modification and roles in the pathology of autophagy-related disorders. Biochimie, 175(Aug), 34–49. https://doi.org/10.1016/j.biochi.2020.04.025.
Rogov, V., Dötsch, V., Johansen, T., & Kirkin, V. (2014). Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Molecular Cell, 53(2), 167–178. https://doi.org/10.1016/j.molcel.2013.12.014.
Bresciani, A., et al. (2018). Quantifying autophagy using novel LC3B and p62 TR-FRET assays. PLoS One, 13(3 Mar), e0194423 https://doi.org/10.1371/journal.pone.0194423.
Finnegan, R. M., Elshazly, A. M., Schoenlein, P. V., & Gewirtz, D. A. (2022). Therapeutic potential for targeting autophagy in ER+ Breast Cancer. Cancers (Basel), 14(17 Sep), 4289. https://doi.org/10.3390/cancers14174289.
Frudd, K., Burgoyne, T., & Burgoyne, J. R. (2018). Oxidation of Atg3 and Atg7 mediates inhibition of autophagy. Nature Communications, 9(1 Jan), 95 https://doi.org/10.1038/s41467-017-02352-z.
Tanida, I. (2011). Autophagy basics. Microbiology Immunology, 55(1 Jan), 1–11. https://doi.org/10.1111/j.1348-0421.2010.00271.x.
Jewell, J. L., & Guan, K.-L. (2013). Nutrient signaling to mTOR and cell growth. Trends in Biochemical Sciences, 38(5 May), 233–242. https://doi.org/10.1016/j.tibs.2013.01.004.
Inoki, K. (2014). mTOR signaling in autophagy regulation in the kidney. Seminars in Nephrology, 34(1 Jan), 2–8. https://doi.org/10.1016/j.semnephrol.2013.11.002.
Yamamoto, H., Zhang, S., & Mizushima, N. (2023). Autophagy genes in biology and disease. Nature Reviews Genetics, 24(6 Jun), 382–400. https://doi.org/10.1038/s41576-022-00562-w.
de Souza, A. S. C., Gonçalves, L. B., Lepique, A. P., & de Araujo-Souza, P. S. (2020) The role of autophagy in tumor immunology—complex mechanisms that may be explored therapeutically. Frontiers in Oncology, 10, Dec., https://doi.org/10.3389/fonc.2020.603661.
Debnath, J., Gammoh, N., & Ryan, K. M. (2023) Autophagy and autophagy-related pathways in cancer, Nature Reviews Molecular Cell Biology, Mar., https://doi.org/10.1038/s41580-023-00585-z.
Laribee, R. N., Boucher, A. B., Madireddy, S., & Pfeffer, L. M. (2023). The STAT3-regulated autophagy pathway in glioblastoma. Pharmaceuticals, 16(5 Apr), 671. https://doi.org/10.3390/ph16050671.
Wang, X., Lee, J., & Xie, C. (2022). Autophagy regulation on cancer stem cell maintenance, metastasis, and therapy resistance. Cancers (Basel), 14(2 Jan), 381 https://doi.org/10.3390/cancers14020381.
Zhang, Y., et al. (2022). Autophagy-related proteins in genome stability: autophagy-dependent and independent actions. International Journal of Biological Sciences, 18(14), 5329–5344. https://doi.org/10.7150/ijbs.76134.
Ji, S., et al. (2023). Cellular rejuvenation: molecular mechanisms and potential therapeutic interventions for diseases. Signal Transduction and Targeted Therapy, 8(1 Mar), 116 https://doi.org/10.1038/s41392-023-01343-5.
Deretic, V. (2021). Autophagy in inflammation, infection, and immunometabolism. Immunity, 54(3 Mar), 437–453. https://doi.org/10.1016/j.immuni.2021.01.018.
Matsuzawa-Ishimoto, Y., Hwang, S., & Cadwell, K. (2018). Autophagy and Inflammation. Annual Review of Immunology, 36(1 Apr), 73–101. https://doi.org/10.1146/annurev-immunol-042617-053253.
Deretic, V., & Klionsky, D. J. (2018). Autophagy and inflammation: a special review issue. Autophagy, 14(2 Feb), 179–180. https://doi.org/10.1080/15548627.2017.1412229.
Deretic, V., Saitoh, T., & Akira, S. (2013). Autophagy in infection, inflammation and immunity. Nature Reviews Immunology, 13(10 Oct), 722–737. https://doi.org/10.1038/nri3532.
Painter, J. D., Galle-Treger, L., & Akbari, O. (2020) Role of autophagy in lung inflammation, Frontiers in Immunology, 11, Jul., https://doi.org/10.3389/fimmu.2020.01337.
Messer, J. S. (2017). The cellular autophagy/apoptosis checkpoint during inflammation. Cellular and Molecular Life Sciences, 74(7 Apr), 1281–1296. https://doi.org/10.1007/s00018-016-2403-y.
Ryter, S. W., Mizumura, K., & Choi, A. M. K. (2014). The impact of autophagy on cell death modalities. International Journal of Cell Biology, 2014, 1–12. https://doi.org/10.1155/2014/502676.
Racanelli, A. C., Kikkers, S. A., Choi, A. M. K., & Cloonan, S. M. (2018). Autophagy and inflammation in chronic respiratory disease. Autophagy, 14(2 Feb), 221–232. https://doi.org/10.1080/15548627.2017.1389823.
Raudenska, M., Balvan, J., & Masarik, M. (2021). Crosstalk between autophagy inhibitors and endosome-related secretory pathways: a challenge for autophagy-based treatment of solid cancers. Molecular Cancer, 20(1 Oct), 140 https://doi.org/10.1186/s12943-021-01423-6.
Bhattacharya, A., Prakash, Y. S., & Eissa, N. T. (2014). Secretory function of autophagy in innate immune cells. Cell Microbiology, 16(11 Nov), 1637–1645. https://doi.org/10.1111/cmi.12365.
Cybulsky, A. V. (2017). Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nature Reviews Nephrology, 13(11 Nov), 681–696. https://doi.org/10.1038/nrneph.2017.129.
Martinez-Outschoorn, U. E., et al. (2011). Cytokine production and inflammation drive autophagy in the tumor microenvironment. Cell Cycle, 10(11 Jun), 1784–1793. https://doi.org/10.4161/cc.10.11.15674.
Reina-Campos, M., Moscat, J., & Diaz-Meco, M. (2017). Metabolism shapes the tumor microenvironment. Current Opinion in Cell Biology, 48(Oct), 47–53. https://doi.org/10.1016/j.ceb.2017.05.006.
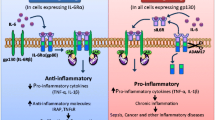
Voronov, E. (2013) Unique versus redundant functions of IL-1α and IL-1β in the tumor microenvironment, Frontiers in Immunology, 4, https://doi.org/10.3389/fimmu.2013.00177.
Bustos, S. O., Antunes, F., Rangel, M. C., & Chammas, R. (2020) Emerging autophagy functions shape the tumor microenvironment and play a role in cancer progression - implications for cancer therapy, Frontiers in Oncology, 10, Nov., https://doi.org/10.3389/fonc.2020.606436.
Dostert, C., Grusdat, M., Letellier, E., & Brenner, D. (2019). The TNF family of ligands and receptors: communication modules in the immune system and beyond. Physiological Reviews, 99(1), 115–160. https://doi.org/10.1152/physrev.00045.2017.
Laha, D., Grant, R., Mishra, P., & Nilubol, N. (2021) The role of tumor necrosis factor in manipulating the immunological response of tumor microenvironment, Frontiers in Immunology, 12, Apr., https://doi.org/10.3389/fimmu.2021.656908.
Sil, S., Niu, F., Tom, E., Liao, K., Periyasamy, P., & Buch, S. (2019). Cocaine mediated neuroinflammation: role of dysregulated autophagy in pericytes. Molecular Neurobiology, 56(5 May), 3576–3590. https://doi.org/10.1007/s12035-018-1325-0.
Kuo, W., Chang, J., Chen, C., Tsao, N., & Chang, C. (2022). Autophagy drives plasticity and functional polarization of tumor‐associated macrophages. IUBMB Life, 74(2 Feb), 157–169. https://doi.org/10.1002/iub.2543.
Abbaszadeh, F., Fakhri, S., & Khan, H. (2020). Targeting apoptosis and autophagy following spinal cord injury: therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacology Research, 160(Oct), 105069. https://doi.org/10.1016/j.phrs.2020.105069.
Hu, F., et al. (2021). IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nature Communications, 12(1 Jun), 3651 https://doi.org/10.1038/s41467-021-23923-1.
Thongchot, S., et al. (2021). Cancer-associated fibroblast-derived IL-6 determines unfavorable prognosis in cholangiocarcinoma by affecting autophagy-associated chemoresponse. Cancers (Basel), 13(9 Apr), 2134. https://doi.org/10.3390/cancers13092134.
Browning, L., Patel, M. R., Horvath, E. B., Tawara, K., & Jorcyk, C. L. (2018). IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Management and Research, 10(Dec), 6685–6693. https://doi.org/10.2147/CMAR.S179189.
Thongchot, S., et al. (2021). Interleukin‑8 released by cancer‑associated fibroblasts attenuates the autophagy and promotes the migration of ovarian cancer cells. International Journal of Oncology, 58(5 Mar), 14. https://doi.org/10.3892/ijo.2021.5194.
Angioni, R., Sánchez-Rodríguez, R., Viola, A., & Molon, B. (2021). TGF-β in cancer: metabolic driver of the tolerogenic crosstalk in the tumor microenvironment. Cancers (Basel), 13(3 Jan), 401 https://doi.org/10.3390/cancers13030401.
du Plessis, M., Davis, T., Loos, B., Pretorius, E., de Villiers, W. J. S., & Engelbrecht, A. M. (2021). Molecular regulation of autophagy in a pro-inflammatory tumour microenvironment: new insight into the role of serum amyloid A. Cytokine & Growth Factor Reviews, 59(Jun), 71–83. https://doi.org/10.1016/j.cytogfr.2021.01.007.
Fuxe, J., & Karlsson, M. C. I. (2012). TGF-β-induced epithelial-mesenchymal transition: a link between cancer and inflammation. Seminars in Cancer Biology, 22(5–6 Oct), 455–461. https://doi.org/10.1016/j.semcancer.2012.05.004.
Wang, J., Li, D., Cang, H., & Guo, B. (2019). Crosstalk between cancer and immune cells: Role of tumor‐associated macrophages in the tumor microenvironment. Cancer Medicine, 8(10 Aug), 4709–4721. https://doi.org/10.1002/cam4.2327.
Wang, W., et al. (2019). Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reproductive Biology and Endocrinology, 17(1 Dec), 2 https://doi.org/10.1186/s12958-018-0444-9.
Gerada, C., & Ryan, K. M. (2020). Autophagy, the innate immune response and cancer. Molecular Oncology, 14(9 Sep), 1913–1929. https://doi.org/10.1002/1878-0261.12774.
Jiang, T., Chen, X., Ren, X., Yang, J.-M., & Cheng, Y. (2021). Emerging role of autophagy in anti-tumor immunity: implications for the modulation of immunotherapy resistance. Drug Resistance Updates, 56(May), 100752 https://doi.org/10.1016/j.drup.2021.100752.
Bernard, A., Jin, M., Xu, Z., & Klionsky, D. J. (2015). A large-scale analysis of autophagy-related gene expression identifies new regulators of autophagy. Autophagy, 11(11 Nov), 2114–2122. https://doi.org/10.1080/15548627.2015.1099796.
Kuo, C.-J., Hansen, M., & Troemel, E. (2018). Autophagy and innate immunity: Insights from invertebrate model organisms. Autophagy, 14(2 Feb), 233–242. https://doi.org/10.1080/15548627.2017.1389824.
Sil, P., Muse, G., & Martinez, J. (2018). A ravenous defense: canonical and non-canonical autophagy in immunity. Current Opinion in Immunology, 50(Feb), 21–31. https://doi.org/10.1016/j.coi.2017.10.004.
Valečka, J., Almeida, C. R., Su, B., Pierre, P., & Gatti, E. (2018). Autophagy and MHC-restricted antigen presentation. Molecular Immunology, 99(Jul), 163–170. https://doi.org/10.1016/j.molimm.2018.05.009.
Van Kaer, L., Parekh, V. V., Postoak, J. L., & Wu, L. (2019). Role of autophagy in MHC class I-restricted antigen presentation. Molecular Immunology, 113(Sep), 2–5. https://doi.org/10.1016/j.molimm.2017.10.021.
Li, M., Tan, J., Miao, Y., Lei, P., & Zhang, Q. (2015). The dual role of autophagy under hypoxia-involvement of interaction between autophagy and apoptosis. Apoptosis, 20(6 Jun), 769–777. https://doi.org/10.1007/s10495-015-1110-8.
Guadamillas, M. C., Cerezo, A., & Del Pozo, M. A. (2011). Overcoming anoikis–pathways to anchorage-independent growth in cancer. Journal of Cell Science, 124(Pt 19 Oct), 3189–3197. https://doi.org/10.1242/jcs.072165.
Adeshakin, F. O., Adeshakin, A. O., Afolabi, L. O., Yan, D., Zhang, G., & Wan, X. (2021) Mechanisms for modulating anoikis resistance in cancer and the relevance of metabolic reprogramming. Frontiers in Oncology, 11, Mar, https://doi.org/10.3389/fonc.2021.626577.
Paoli, P., Giannoni, E., & Chiarugi, P. (2013). Anoikis molecular pathways and its role in cancer progression. Biochimica et Biophysica Acta - Molecular Cell Research, 1833(12 Dec), 3481–3498. https://doi.org/10.1016/j.bbamcr.2013.06.026.
Malagobadan, S., & Nagoor, H. (2018) Anoikis, https://doi.org/10.1016/B978-0-12-801238-3.65021-3.
Warren, C. F. A., Wong-Brown, M. W., & Bowden, N. A. (2019). BCL-2 family isoforms in apoptosis and cancer. Cell Death & Disease, 10(3 Feb), 177 https://doi.org/10.1038/s41419-019-1407-6.
Tan, K., Goldstein, D., Crowe, P., & Yang, J.-L. (2013). Uncovering a key to the process of metastasis in human cancers: a review of critical regulators of anoikis. Journal of Cancer Research and Clinical Oncology, 139(11 Nov), 1795–1805. https://doi.org/10.1007/s00432-013-1482-5.
Shamas-Din, A., Kale, J., Leber, B., & Andrews, D. W. (2013). Mechanisms of action of Bcl-2 family proteins. Cold Spring Harbor Perspectives in Biology, 5(4 Apr), a008714–a008714. https://doi.org/10.1101/cshperspect.a008714.
Shahar, N., & Larisch, S. (2020). Inhibiting the inhibitors: targeting anti-apoptotic proteins in cancer and therapy resistance. Drug Resistance Updates, 52(Sep), 100712 https://doi.org/10.1016/j.drup.2020.100712.
Wei, Y. et al. (2020) Targeting Bcl-2 proteins in acute myeloid leukemia. Frontiers in Oncology, 10, Nov. https://doi.org/10.3389/fonc.2020.584974.
Kapoor, I., Bodo, J., Hill, B. T., Hsi, E. D., & Almasan, A. (2020). Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance. Cell Death & Disease, 11(11 Nov), 941 https://doi.org/10.1038/s41419-020-03144-y.
Hafezi, S., & Rahmani, M. (2021). Targeting BCL-2 in cancer: advances, challenges, and perspectives. Cancers (Basel), 13(6 Mar), 1292 https://doi.org/10.3390/cancers13061292.
Knight, T., Luedtke, D., Edwards, H., Taub, J. W., & Ge, Y. (2019). A delicate balance – The BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochemical Pharmacology, 162(Apr), 250–261. https://doi.org/10.1016/j.bcp.2019.01.015.
Lopez, A., et al. (2022). Co-targeting of BAX and BCL-XL proteins broadly overcomes resistance to apoptosis in cancer. Nature Communications, 13(1 Mar), 1199 https://doi.org/10.1038/s41467-022-28741-7.
Sarosiek, K. A., & Wood, K. C. (2023). Endogenous and imposed determinants of apoptotic vulnerabilities in cancer. Trends in Cancer, 9(2 Feb), 96–110. https://doi.org/10.1016/j.trecan.2022.10.004.
Carneiro, B. A., & El-Deiry, W. S. (2020). Targeting apoptosis in cancer therapy. Nature Reviews Clinical Oncology, 17(7 Jul), 395–417. https://doi.org/10.1038/s41571-020-0341-y.
Zaman, S., Wang, R., & Gandhi, V. (2014). Targeting the apoptosis pathway in hematologic malignancies. Leukemia & Lymphoma, 55(9 Sep), 1980–1992. https://doi.org/10.3109/10428194.2013.855307.
McIlwain, D. R., Berger, T., & Mak, T. W. (2013). Caspase functions in cell death and disease. Cold Spring Harbor Perspectives in Biology, 5(4 Apr), a008656–a008656. https://doi.org/10.1101/cshperspect.a008656.
Kim, Y.-N., Koo, K. H., Sung, J. Y., Yun, U.-J., & Kim, H. (2012). Anoikis resistance: an essential prerequisite for tumor metastasis. International Journal of Cell Biology, 2012, 1–11. https://doi.org/10.1155/2012/306879.
Raeisi, M., Zehtabi, M., Velaei, K., Fayyazpour, P., Aghaei, N., & Mehdizadeh, A. (2022). Anoikis in cancer: the role of lipid signaling. Cell Biology International, 46(11 Nov), 1717–1728. https://doi.org/10.1002/cbin.11896.
Geissler, A., et al. (2013). Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK. Cell Death Differ, 20(10 Oct), 1317–1329. https://doi.org/10.1038/cdd.2013.78.
Siddiqui, W. A., Ahad, A., & Ahsan, H. (2015). The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Archives of Toxicology, 89(3 Mar), 289–317. https://doi.org/10.1007/s00204-014-1448-7.
Cairns, R. A., Harris, I. S., & Mak, T. W. (2011). Regulation of cancer cell metabolism. Nature Reviews Cancer, 11(2 Feb), 85–95. https://doi.org/10.1038/nrc2981. Nature Publishing Group.
Green, D. R. (2022). The mitochondrial pathway of apoptosis Part II: The BCL-2 protein family. Cold Spring Harbor Perspectives in Biology, 14(6 Jun), a041046 https://doi.org/10.1101/cshperspect.a041046.
Popgeorgiev, N., et al. (2020). Ancient and conserved functional interplay between Bcl-2 family proteins in the mitochondrial pathway of apoptosis. Science Advances, 6(Oct), 40. https://doi.org/10.1126/sciadv.abc4149.
Peyre, L., Meyer, M., Hofman, P., & Roux, J. (2021). TRAIL receptor-induced features of epithelial-to-mesenchymal transition increase tumour phenotypic heterogeneity: potential cell survival mechanisms. British Journal of Cancer, 124(1 Jan), 91–101. https://doi.org/10.1038/s41416-020-01177-w.
Huang, J., Yu, S., Ji, C., & Li, J. (2015). Structural basis of cell apoptosis and necrosis in TNFR signaling. Apoptosis, 20(2 Feb), 210–215. https://doi.org/10.1007/s10495-014-1061-5.
Kantari, C., & Walczak, H. (2011). Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Biochimica et Biophysica Acta - Molecular Cell Research, 1813(4 Apr), 558–563. https://doi.org/10.1016/j.bbamcr.2011.01.026.
Ding, L., et al. (2011). Cisplatin restores TRAIL apoptotic pathway in glioblastoma-derived stem cells through up-regulation of DR5 and down-regulation of c-flip. Cancer Investigation, 29(8), 511–520. https://doi.org/10.3109/07357907.2011.605412.
Ivanisenko, N. V., et al. (2022). Regulation of extrinsic apoptotic signaling by c-FLIP: towards targeting cancer networks. Trends in Cancer, 8(3 Mar), 190–209. https://doi.org/10.1016/j.trecan.2021.12.002.
Bergers, G., & Fendt, S.-M. (2021). The metabolism of cancer cells during metastasis. Nature Reviews Cancer, 21(3 Mar), 162–180. https://doi.org/10.1038/s41568-020-00320-2.
Janiszewska, M., Primi, M. C., & Izard, T. (2020). Cell adhesion in cancer: beyond the migration of single cells. Journal of Biological Chemistry, 295(8 Feb), 2495–2505. https://doi.org/10.1074/jbc.REV119.007759.
Kadry, Y. A., & Calderwood, D. A. (2020). Chapter 22: Structural and signaling functions of integrins. Biochimica et Biophysica Acta - Biomembranes, 1862(5 May), 183206 https://doi.org/10.1016/j.bbamem.2020.183206.
Pang, X., et al. (2023). Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduction and Targeted Therapy, 8(1 Jan), 1. https://doi.org/10.1038/s41392-022-01259-6.
Vachon, P. H. (2011) Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. Journal of Signal Transduction, 2011, https://doi.org/10.1155/2011/738137.
Gahmberg, C. G., Fagerholm, S. C., Nurmi, S. M., Chavakis, T., Marchesan, S., & Grönholm, M. (2009). Regulation of integrin activity and signalling. Biochimica et Biophysica Acta - General Subjects, 1790(6), 431–444. https://doi.org/10.1016/j.bbagen.2009.03.007.
Desgrosellier, J. S., & Cheresh, D. A. (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nature Reviews Cancer, 10(1 Jan), 9–22. https://doi.org/10.1038/nrc2748.
Revathidevi, S., & Munirajan, A. K. (2019). Akt in cancer: mediator and more. Seminars in Cancer Biology, 59(Dec), 80–91. https://doi.org/10.1016/j.semcancer.2019.06.002.
Morana, O., Wood, W., & Gregory, C. D. (2022). The apoptosis paradox in cancer. International Journal of Molecular Science, 23(3 Jan), 1328 https://doi.org/10.3390/ijms23031328.
Kesavardhana, S., Malireddi, R. K. S., & Kanneganti, T.-D. (2020). Caspases in cell death, inflammation, and pyroptosis. Annual Review of Immunology, 38(Apr), 567–595. https://doi.org/10.1146/annurev-immunol-073119-095439.
Nirmala, J. G., & Lopus, M. (2020). Cell death mechanisms in eukaryotes. Cell Biology and Toxicology, 36(2), 145–164. https://doi.org/10.1007/s10565-019-09496-2.
Zhou, M., et al. (2015). Atomic structure of the apoptosome: mechanism of cytochrome c - and dATP-mediated activation of Apaf-1. Genes & Development, 29(22 Nov), 2349–2361. https://doi.org/10.1101/gad.272278.115.
Lossi, L. (2022). The concept of intrinsic versus extrinsic apoptosis. Biochemical Journal, 479(3 Feb), 357–384. https://doi.org/10.1042/BCJ20210854.
Whelan, K. A., et al. (2010). Hypoxia suppression of Bim and Bmf blocks anoikis and luminal clearing during mammary morphogenesis. Molecular Biology of the Cell, 21(22 Nov), 3829–3837. https://doi.org/10.1091/mbc.e10-04-0353.
Urade, R. et al. (2023) A fluorene derivative inhibits human hepatocellular carcinoma cells by ROS-mediated apoptosis, anoikis and autophagy. Life Sciences, 121835, Jun., https://doi.org/10.1016/j.lfs.2023.121835.
Zhao, G.-S., et al. (2019). High expression of ID1 facilitates metastasis in human osteosarcoma by regulating the sensitivity of anoikis via PI3K/AKT depended suppression of the intrinsic apoptotic signaling pathway. American Journal of Translational Research, 11(4), 2117–2139. http://www.ncbi.nlm.nih.gov/pubmed/31105823.
Guha, D., et al. (2019). Integrin-EGFR interaction regulates anoikis resistance in colon cancer cells. Apoptosis, 24(11–12 Dec), 958–971. https://doi.org/10.1007/s10495-019-01573-5.
Vlahakis, A., & Debnath, J. (2017). The interconnections between autophagy and integrin-mediated cell adhesion. Journal of Molecular Biology, 429(4 Feb), 515–530. https://doi.org/10.1016/j.jmb.2016.11.027.
Elmore, S. (2007). Apoptosis: a review of programmed cell death. Toxicologic Pathology, 35(4 Jun), 495–516. https://doi.org/10.1080/01926230701320337.
Roy, S. K., Srivastava, R. K., & Shankar, S. (2010). Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. Journal of Molecular Signaling, 5(Jul), 10 https://doi.org/10.1186/1750-2187-5-10.
Fujimoto, N., Gemba, K., & Kishimoto, T. (2011). Clinical significance of serum vascular endothelial growth factor in malignant pleural mesothelioma. Journal of Thoracic Oncology, 6(5 May), 971–972. https://doi.org/10.1097/JTO.0b013e318215a384. Elsevier.
Cheng, D., Liang, B., & Kong, H. (2012). Clinical significance of vascular endothelial growth factor and endostatin levels in the differential diagnosis of malignant and benign ascites. Medical Oncology, 29(2 Jun), 1397–1402. https://doi.org/10.1007/s12032-011-9972-2.
Singh, R., Letai, A., & Sarosiek, K. (2019). Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nature Reviews Molecular Cell Biology, 20(3), 175–193. https://doi.org/10.1038/s41580-018-0089-8.
Agena, R., de Jesús Cortés-Sánchez, A., Hernández-Sánchez, H., & Jaramillo-Flores, M. E. (2023). Pro-apoptotic activity of bioactive compounds from seaweeds: promising sources for developing novel anticancer drugs. Marine Drugs, 21(3 Mar), 182 https://doi.org/10.3390/md21030182.
Lotti, R., et al. (2010). A previously unreported function of β1B integrin isoform in caspase-8-dependent integrin-mediated keratinocyte death. Journal of Investigative Dermatology, 130(11 Nov), 2569–2577. https://doi.org/10.1038/jid.2010.195.
Lindsey, S., & Langhans, S. A. Epidermal Growth Factor Signaling in Transformed Cells, 2015, 1–41. https://doi.org/10.1016/bs.ircmb.2014.10.001.
Geiger, T. R., & Peeper, D. S. (2007). Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Research, 67(13 Jul), 6221–6229. https://doi.org/10.1158/0008-5472.CAN-07-0121.
Yu, X., Liu, L., Cai, B., He, Y., & Wan, X. (2008). Suppression of anoikis by the neurotrophic receptor TrkB in human ovarian cancer. Cancer Science, 99(3 Mar), 543–552. https://doi.org/10.1111/j.1349-7006.2007.00722.x.
Geiger, T. R., & Peeper, D. S. (2005) The neurotrophic receptor TrkB in anoikis resistance and metastasis: a perspective. https://doi.org/10.1158/0008-5472.CAN-05-0709.
Dongre, A., & Weinberg, R. A. (2019). New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nature Reviews Molecular Cell Biology, 20(2 Feb), 69–84. https://doi.org/10.1038/s41580-018-0080-4.
Yang, J., et al. (2020). Guidelines and definitions for research on epithelial–mesenchymal transition. Nature Reviews Molecular Cell Biology, 21(6 Jun), 341–352. https://doi.org/10.1038/s41580-020-0237-9.
Nieto, M. A. (2013). Epithelial plasticity: a common theme in embryonic and cancer cells. Science (80-), 342(Nov), 6159. https://doi.org/10.1126/science.1234850.
Aiello, N. M., et al. (2018). EMT subtype influences epithelial plasticity and mode of cell migration. Developmental Cell, 45(6 Jun), 681–695.e4. https://doi.org/10.1016/j.devcel.2018.05.027.
Nakaya, Y., & Sheng, G. (2013). EMT in developmental morphogenesis. Cancer Letters, 341(1 Nov), 9–15. https://doi.org/10.1016/j.canlet.2013.02.037.
Chen, T., You, Y., Jiang, H., & Wang, Z. Z. (2017). Epithelial-mesenchymal transition (EMT): a biological process in the development, stem cell differentiation, and tumorigenesis. Journal of Cellular Physiology, 232(12 Dec), 3261–3272. https://doi.org/10.1002/jcp.25797.
Terry, S., et al. (2017). New insights into the role of EMT in tumor immune escape. Molecular Oncology, 11(7 Jul), 824–846. https://doi.org/10.1002/1878-0261.12093.
Zhong, W., & Sun, T. (2023) Editorial: epithelial-mesenchymal transition (EMT) as a therapeutic target in cancer, Frontiers in Oncology, 13, Jan., https://doi.org/10.3389/fonc.2023.1121416.
Malagoli Tagliazucchi, G., Wiecek, A. J., Withnell, E., & Secrier, M. (2023). Genomic and microenvironmental heterogeneity shaping epithelial-to-mesenchymal trajectories in cancer. Nature Communications, 14(1 Feb), 789 https://doi.org/10.1038/s41467-023-36439-7.
Chou, M.-Y., & Yang, M.-H. (2021). Interplay of immunometabolism and epithelial–mesenchymal transition in the tumor microenvironment. International Journal of Molecular Science, 22(18 Sep), 9878 https://doi.org/10.3390/ijms22189878.
Li, D., et al. (2023). Heterogeneity and plasticity of epithelial–mesenchymal transition (EMT) in cancer metastasis: focusing on partial EMT and regulatory mechanisms. Cell Proliferation, 56(Jun), 6 https://doi.org/10.1111/cpr.13423.
Hapke, R. Y., & Haake, S. M. (2020). Hypoxia-induced epithelial to mesenchymal transition in cancer. Cancer Letters, 487(Sep), 10–20. https://doi.org/10.1016/j.canlet.2020.05.012.
Wang, Y., Shi, J., Chai, K., Ying, X., & Zhou, B. (2013). The role of snail in EMT and tumorigenesis. Current Cancer Drug Targets, 13(9 Dec), 963–972. https://doi.org/10.2174/15680096113136660102.
Tiwari, I., Yoon, M.-H., Park, B.-J., & Jang, K. L. (2015). Hepatitis C virus core protein induces epithelial–mesenchymal transition in human hepatocytes by upregulating E12/E47 levels. Cancer Letters, 362(1 Jun), 131–138. https://doi.org/10.1016/j.canlet.2015.03.032.
Smit, M. A., & Peeper, D. S. (2011). Zeb1 is required for TrkB-induced epithelial-mesenchymal transition, anoikis resistance and metastasis. Oncogene, 30(35 Sep), 3735–3744. https://doi.org/10.1038/onc.2011.96.
Kumar, S., et al. (2011). A pathway for the control of anoikis sensitivity by E-Cadherin and epithelial-to-mesenchymal transition. Molecular and Cellular Biology, 31(19 Oct), 4036–4051. https://doi.org/10.1128/mcb.01342-10.
Salvi, A. M., Bays, J. L., Mackin, S. R., Mege, R.-M., & DeMali, K. A. (2021). Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake. Nature Cell Biology, 23(5 May), 457–466. https://doi.org/10.1038/s41556-021-00677-y.
Cadwell, C. M., Su, W., & Kowalczyk, A. P. (2016). Cadherin tales: regulation of cadherin function by endocytic membrane trafficking. Traffic, 17(12 Dec), 1262–1271. https://doi.org/10.1111/tra.12448.
Jenkins, P. M., Vasavda, C., Hostettler, J., Davis, J. Q., Abdi, K., & Bennett, V. (2013). E-cadherin polarity is determined by a multifunction motif mediating lateral membrane retention through Ankyrin-G and apical-lateral transcytosis through clathrin. Journal of Biological Chemistry, 288(20 May), 14018–14031. https://doi.org/10.1074/jbc.M113.454439.
Frisch, S. M., Schaller, M., & Cieply, B. (2013). Mechanisms that link the oncogenic epithelial–mesenchymal transition to suppression of anoikis. Journal of Cell Science, 126(1 Jan), 21–29. https://doi.org/10.1242/jcs.120907.
Loh, C.-Y., et al. (2019). The E-Cadherin and N-Cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells, 8(10 Sep), 1118 https://doi.org/10.3390/cells8101118.
Aleskandarany, M. A., et al. (2014). Epithelial mesenchymal transition in early invasive breast cancer: an immunohistochemical and reverse phase protein array study. Breast Cancer Research and Treatment, 145(2 Jun), 339–348. https://doi.org/10.1007/s10549-014-2927-5.
Mylavarapu, S. et al., Activation of epithelial-mesenchymal transition and altered β-catenin signaling in a novel Indian colorectal carcinoma cell line. Frontiers in Oncology, 9. 2019. [Online]. Available: https://doi.org/10.3389/fonc.2019.00054.
Simpson, C. D., Anyiwe, K., & Schimmer, A. D. (2008). Anoikis resistance and tumor metastasis. Cancer Letters, 272(2 Dec), 177–185. https://doi.org/10.1016/j.canlet.2008.05.029. Elsevier Ireland Ltd.
Liu, H., Ong, S.-E., Badu-Nkansah, K., Schindler, J., White, F. M., & Hynes, R. O. CUB-domain-containing protein 1 (CDCP1) activates Src to promote melanoma metastasis. Proceedings of the National Academy of Sciences of the United States of America, https://doi.org/10.1073/pnas.1017228108.
Uekita, T., & Sakai, R. (2011). Roles of CUB domain-containing protein 1 signaling in cancer invasion and metastasis. Wiley Online Library, 102(11 Nov), 1943–1948. https://doi.org/10.1111/j.1349-7006.2011.02052.x.
González-Llorente, L., et al. (2020). Overexpression of mitochondrial if1 prevents metastatic disease of colorectal cancer by enhancing anoikis and tumor infiltration of NK cells. Cancers (Basel), 12(1), 6–8. https://doi.org/10.3390/cancers12010022.
Oh, Y.-T., & Sun, S.-Y. (2021). Regulation of cancer metastasis by TRAIL/death receptor signaling. Biomolecules, 11(4 Mar), 499. https://doi.org/10.3390/biom11040499.
Baulida, J. (2017). Epithelial-to-mesenchymal transition transcription factors in cancer-associated fibroblasts. Molecular Oncology, 11(7 Jul), 847–859. https://doi.org/10.1002/1878-0261.12080.
Maurizi, A., Ciocca, M., Giuliani, C., Di Carlo, I., & Teti, A. (2022). Role of neural (N)-cadherin in breast cancer cell stemness and dormancy in the bone microenvironment. Cancers (Basel), 14(5 Mar), 1317 https://doi.org/10.3390/cancers14051317.
van Roy, F. (2014). Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nature Reviews Cancer, 14(2 Feb), 121–134. https://doi.org/10.1038/nrc3647.
Qi, Z., & Chen, L. Endoplasmic reticulum stress and autophagy, 2019, 167–177. https://doi.org/10.1007/978-981-15-0602-4_8.
Kegelman, T. P., et al. (2017). Inhibition of radiation-induced glioblastoma invasion by genetic and pharmacological targeting of MDA-9/Syntenin. Proceedings of the National Academy of Sciences of the United States of America, 114(2 Jan), 370–375. https://doi.org/10.1073/pnas.1616100114.
Talukdar, S., et al. (2018). MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proceedings of the National Academy of Sciences of the United States of America, 115(22 May), 5768–5773. https://doi.org/10.1073/pnas.1721650115.
Chaurasia, M., Bhatt, A. N., Das, A., Dwarakanath, B. S., & Sharma, K. (2016). Radiation-induced autophagy: mechanisms and consequences. Free Radical Research, 50(3 Mar), 273–290. https://doi.org/10.3109/10715762.2015.1129534.
Sun, K., et al. (2013) Paradoxical roles of autophagy in different stages of tumorigenesis: protector for normal or cancer cells, Cell and Bioscience 3, 1. BioMed Central, 35, Sep. https://doi.org/10.1186/2045-3701-3-35.
Yu, Y., et al. (2022). ATF4/CEMIP/PKCα promotes anoikis resistance by enhancing protective autophagy in prostate cancer cells. Cell Death & Disease, 13(1), 46 https://doi.org/10.1038/s41419-021-04494-x.
Dasgupta, S., et al. (2013). Human Cancer Biology novel role of MDA-9/Syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clinical Cancer Research, 19, 17 https://doi.org/10.1158/1078-0432.CCR-13-0585.
Pradhan, A. K., Maji, S., Das, S. K., Emdad, L., Sarkar, D., & Fisher, P. B. (2020). MDA-9/Syntenin/SDCBP: new insights into a unique multifunctional scaffold protein. Cancer and Metastasis Reviews, 39(3 Sep), 769–781. https://doi.org/10.1007/s10555-020-09886-7.
Talukdar, S. et al. MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells, Proceedings of the National Academy of Sciences of the United States of America, https://doi.org/10.1073/pnas.1721650115.
Mukhopadhyay, S., Mahapatra, K. K., Praharaj, P. P., Patil, S., & Bhutia, S. K. (2022). Recent progress of autophagy signaling in tumor microenvironment and its targeting for possible cancer therapeutics. Seminars in Cancer Biology, 85(Oct), 196–208. https://doi.org/10.1016/j.semcancer.2021.09.003.
Gillson, J., et al. (2022). Autophagy: a key player in pancreatic cancer progression and a potential drug target. Cancers (Basel), 14(14 Jul), 3528. https://doi.org/10.3390/cancers14143528.
Michalkova, R., Mirossay, L., Gazdova, M., Kello, M., & Mojzis, J. (2021). Molecular mechanisms of antiproliferative effects of natural chalcones. Cancers (Basel), 13(11 May), 2730. https://doi.org/10.3390/cancers13112730.
Kageyama, S., et al. (2021). p62/SQSTM1-droplet serves as a platform for autophagosome formation and anti-oxidative stress response. Nature Communications, 12(1 Jan), 16 https://doi.org/10.1038/s41467-020-20185-1.
Lippai, M., & Lőw, P. (2014). The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. BioMed Research International, 2014, 1–11. https://doi.org/10.1155/2014/832704.
Noda, N. N., & Inagaki, F. (2015). Mechanisms of autophagy. Annual Review of Biophysics, 44(1 Jun), 101–122. https://doi.org/10.1146/annurev-biophys-060414-034248.
Gross, A. S., & Graef, M. (2020). Mechanisms of autophagy in metabolic stress response. Journal of Molecular Biology, 432(1 Jan), 28–52. https://doi.org/10.1016/j.jmb.2019.09.005.
Mizushima, N. (2020). The ATG conjugation systems in autophagy. Current Opinion in Cell Biology, 63(Apr), 1–10. https://doi.org/10.1016/j.ceb.2019.12.001.
Majeed, S. T., Majeed, R., & Andrabi, K. I. (2022). Expanding the view of the molecular mechanisms of autophagy pathway. Journal of Cellular Physiology, 237(8 Aug), 3257–3277. https://doi.org/10.1002/jcp.30819.
Yu, L., Chen, Y., & Tooze, S. A. (2018). Autophagy pathway: cellular and molecular mechanisms. Autophagy, 14(2 Feb), 207–215. https://doi.org/10.1080/15548627.2017.1378838.
Kirkin, V. (2020). History of the selective autophagy research: how did it begin and where does it stand today? Journal of Molecular Biology, 432(1 Jan), 3–27. https://doi.org/10.1016/j.jmb.2019.05.010.
Chourasia, A. H., Boland, M. L., & Macleod, K. F. (2015). Mitophagy and cancer. Cancer & Metabolism, 3(Dec), 1. https://doi.org/10.1186/s40170-015-0130-8.
Onishi, M., Yamano, K., Sato, M., Matsuda, N., & Okamoto, K. (2021). Molecular mechanisms and physiological functions of mitophagy. The EMBO Journal, 40(Feb), 3. https://doi.org/10.15252/embj.2020104705.
Panigrahi, D. P., et al. (2020). The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Seminars in Cancer Biology, 66(Nov), 45–58. https://doi.org/10.1016/j.semcancer.2019.07.015.
Poole, L. P., & Macleod, K. F. (2021). Mitophagy in tumorigenesis and metastasis. Cellular and Molecular Life Sciences, 78(8 Apr), 3817–3851. https://doi.org/10.1007/s00018-021-03774-1.
Yang, X., Pan, W., Xu, G., & Chen, L. (2020). Mitophagy: a crucial modulator in the pathogenesis of chronic diseases. Clinica Chimica Acta, 502(Mar), 245–254. https://doi.org/10.1016/j.cca.2019.11.008.
Zhang, T., Liu, Q., Gao, W., Sehgal, S. A., & Wu, H. (2022). The multifaceted regulation of mitophagy by endogenous metabolites. Autophagy, 18(6 Jun), 1216–1239. https://doi.org/10.1080/15548627.2021.1975914.
Zhang, J., et al. (2021). PINK1/PARK2 dependent mitophagy effectively suppresses NLRP3 inflammasome to alleviate acute pancreatitis. Free Radical Biology and Medicine, 166(Apr), 147–164. https://doi.org/10.1016/j.freeradbiomed.2021.02.019.
Roperto, S., De Falco, F., Perillo, A., Catoi, C., & Roperto, F. (2019). Mitophagy mediated by BNIP3 and BNIP3L/NIX in urothelial cells of the urinary bladder of cattle harbouring bovine papillomavirus infection. Veterinary Microbiology, 236(Sep), 108396 https://doi.org/10.1016/j.vetmic.2019.108396.
Monaci, S., et al. (2021). Hypoxia enhances the expression of RNASET2 in human monocyte-derived dendritic cells: role of PI3K/AKT pathway. International Journal of Molecular Science, 22(14 Jul), 7564 https://doi.org/10.3390/ijms22147564.
Escamilla-Ramírez, A., et al. (2020). Autophagy as a potential therapy for malignant glioma. Pharmaceuticals, 13(7 Jul), 156 https://doi.org/10.3390/ph13070156.
Liu, A., Li, Y., Shen, L., Li, N., Shen, L., & Li, Z. (2022). Pan-cancer analysis of a novel indicator of necroptosis with its application in human cancer. Aging (Albany. NY), 14(18 Sep), 7587–7616. https://doi.org/10.18632/aging.204307.
Liu, H., et al. (2022). The role of FUNDC1 in mitophagy, mitochondrial dynamics and human diseases. Biochemical Pharmacology, 197(Mar), 114891. https://doi.org/10.1016/j.bcp.2021.114891.
Yang, A., et al. (2021). Melatonin inhibits triple-negative breast cancer progression through the Lnc049808-FUNDC1 pathway. Cell Death & Disease, 12(8 Jul), 712 https://doi.org/10.1038/s41419-021-04006-x.
Zhang, W. (2021). The mitophagy receptor FUN14 domain-containing 1 (FUNDC1): a promising biomarker and potential therapeutic target of human diseases. Genes & Disease, 8(5 Sep), 640–654. https://doi.org/10.1016/j.gendis.2020.08.011.
Kongara, S., & Karantza, V. (2012) The interplay between autophagy and ROS in tumorigenesis, Frontiers in Oncology, 2. [Online]. Available: https://doi.org/10.3389/fonc.2012.00171.
Chaurasia, M., Misra, S., Bhatt, A. N., Das, A., Dwarakanath, B., & Sharma, K. (2015). Metabolic imbalance associated mitophagy in tumor cells: genesis and implications. Journal of Cancer Research Updates, 4(2 Apr), 95–107. https://doi.org/10.6000/1929-2279.2015.04.02.8.
Gundamaraju, R., et al. (2022). Autophagy and EMT in cancer and metastasis: who controls whom? Biochimica et Biophysica Acta - Molecular Basis of Disease, 1868(9 Sep), 166431. https://doi.org/10.1016/j.bbadis.2022.166431.
Ashkenazi, A., & Salvesen, G. (2014). Regulated cell death: signaling and mechanisms. Annual Review of Cell and Developmental Biology, 30(1 Oct), 337–356. https://doi.org/10.1146/annurev-cellbio-100913-013226.
Yaacoub, K., Pedeux, R., Tarte, K., & Guillaudeux, T. (2016). Role of the tumor microenvironment in regulating apoptosis and cancer progression. Cancer Letters, 378(2 Aug), 150–159. https://doi.org/10.1016/j.canlet.2016.05.012.
Jing, X., et al. (2019). Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Molecular Cancer, 18(1 Dec), 157. https://doi.org/10.1186/s12943-019-1089-9.
Diao, X., Guo, C., & Li, S. (2023). Identification of a novel anoikis‐related gene signature to predict prognosis and tumor microenvironment in lung adenocarcinoma. Thoracic Cancer, 14(3 Jan), 320–330. https://doi.org/10.1111/1759-7714.14766.
Chavez-Dominguez, R., Perez-Medina, M., Lopez-Gonzalez, J. S., Galicia-Velasco, M., & Aguilar-Cazares, D. (2020) The double-edge sword of autophagy in cancer: from tumor suppression to pro-tumor activity. Frontiers in Oncology, 10, Oct., https://doi.org/10.3389/fonc.2020.578418.
La Belle Flynn, A., & Schiemann, W. P. (2019) Autophagy in breast cancer metastatic dormancy: tumor suppressing or tumor promoting functions? Journal of Cancer Metastasis and Treatment, 2019, May, https://doi.org/10.20517/2394-4722.2019.13.
Liao, M., et al. (2022). Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies. Journal of Hematology & Oncology, 15(1 Apr), 44. https://doi.org/10.1186/s13045-022-01260-0.
Xu, H.-D., & Qin, Z.-H. Beclin 1, Bcl-2 and Autophagy, 2019, 109–126. https://doi.org/10.1007/978-981-15-0602-4_5.
Wang, P., et al. (2019). ROS -mediated p53 activation by juglone enhances apoptosis and autophagy in vivo and in vitro. Toxicology and Applied Pharmacology, 379(Sep), 114647. https://doi.org/10.1016/j.taap.2019.114647.
Pisani, C., et al. (2020). Apoptotic and predictive factors by Bax, Caspases 3/9, Bcl-2, p53 and Ki-67 in prostate cancer after 12 Gy single-dose. Science Reports, 10(1), 7050. https://doi.org/10.1038/s41598-020-64062-9.
Kim, S. M., et al. (2020). Sinensetin induces autophagic cell death through p53-related AMPK/mTOR signaling in hepatocellular carcinoma HepG2 cells. Nutrients, 12(8 Aug), 2462. https://doi.org/10.3390/nu12082462.
Lee, Y., & Kwon, Y. H. (2019). Regulation of apoptosis and autophagy by luteolin in human hepatocellular cancer Hep3B cells. Biochemical and Biophysical Research Communications, 517(4 Oct), 617–622. https://doi.org/10.1016/j.bbrc.2019.07.073.
Biray Avci, C., Sezgin, B., Goker Bagca, B., Karci, H. B., & Gode, S. (2020). PI3K/AKT/mTOR pathway and autophagy regulator genes in paranasal squamous cell carcinoma metastasis. Molecular Biology Reports, 47(5 May), 3641–3651. https://doi.org/10.1007/s11033-020-05458-8.
Xu, W., Yu, M., Qin, J., Luo, Y., & Zhong, M. (2020). LACTB regulates PIK3R3 to promote autophagy and inhibit EMT and proliferation through the PI3K/AKT/mTOR signaling pathway in colorectal cancer. Cancer Management and Research, 12(Dec), 5181–5200. https://doi.org/10.2147/CMAR.S250661.
Braicu, C., et al. (2022). Natural compounds modulate the crosstalk between apoptosis- and autophagy-regulated signaling pathways: controlling the uncontrolled expansion of tumor cells. Seminars in Cancer Biology, 80(May), 218–236. https://doi.org/10.1016/j.semcancer.2020.05.015.
Zhang, Q., et al. (2020). Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomedicine & Pharmacotherapy, 128(Aug), 110245 https://doi.org/10.1016/j.biopha.2020.110245.
Han, Z., et al. (2021). PNO1 regulates autophagy and apoptosis of hepatocellular carcinoma via the MAPK signaling pathway. Cell Death & Disease, 12(6 May), 552 https://doi.org/10.1038/s41419-021-03837-y.
Nguyen, H.-N., et al. (2014). Engineering ePTEN, an enhanced PTEN with increased tumor suppressor activities. Proceedings of the National Academy of Sciences of the United States of America, 111(Jul), 26 https://doi.org/10.1073/pnas.1409433111.
Shu, F., et al. (2023). Epigenetic and post-translational modifications in autophagy: biological functions and therapeutic targets. Signal Transductuction and Targeted Therapy, 8(1 Jan), 32 https://doi.org/10.1038/s41392-022-01300-8.
Rakesh, R., PriyaDharshini, L. C., Sakthivel, K. M., & Rasmi, R. R. (2022). Role and regulation of autophagy in cancer. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1868(7 Jul), 166400 https://doi.org/10.1016/j.bbadis.2022.166400.
Vega-Rubín-de-Celis, S., Kinch, L., & Peña-Llopis, S. (2020). Regulation of Beclin 1-mediated autophagy by oncogenic tyrosine kinases. International Journal of Molecular Science, 21(23 Dec), 9210 https://doi.org/10.3390/ijms21239210.
Hill, S. M., Wrobel, L., & Rubinsztein, D. C. (2019). Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation. Cell Death Differ, 26(4 Apr), 617–629. https://doi.org/10.1038/s41418-018-0254-9.
Marquez, R. T., & Xu, L. Bcl-2:Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch.
Decuypere, J.-P., Parys, J. B., & Bultynck, G. (2012). Regulation of the autophagic Bcl-2/Beclin 1 Interaction. Cells, 1(3 Jul), 284–312. https://doi.org/10.3390/cells1030284.
Das, S., Shukla, N., Singh, S. S., Kushwaha, S., & Shrivastava, R. (2021). Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis, 26(9–10 Oct), 512–533. https://doi.org/10.1007/s10495-021-01687-9.
Li, S., et al. (2019). Shear stress promotes anoikis resistance of cancer cells via caveolin‐1‐dependent extrinsic and intrinsic apoptotic pathways. Journal of Cellular Physiology, 234(4 Apr), 3730–3743. https://doi.org/10.1002/jcp.27149.
Dower, C. M., Wills, C. A., Frisch, S. M., & Wang, H.-G. (2018). Mechanisms and context underlying the role of autophagy in cancer metastasis. Autophagy, 14(7 Jul), 1110–1128. https://doi.org/10.1080/15548627.2018.1450020.
Xu, H.-D., et al. (2013). The pro-survival role of autophagy depends on Bcl-2 under nutrition stress conditions. PLoS One, 8(5 May), e63232. https://doi.org/10.1371/journal.pone.0063232.
Wang, D., Berglund, A., Kenchappa, R. S., Forsyth, P. A., Mulé, J. J., & Etame, A. B. (2016). BIRC3 is a novel driver of therapeutic resistance in Glioblastoma. Science Reports, 6(1), 21710. https://doi.org/10.1038/srep21710.
Kim, J., Chee, W.-Y., Yabuta, N., Kajiwara, K., Nada, S., & Okada, M. (2020). Atg5-mediated autophagy controls apoptosis/anoikis via p53/Rb pathway in naked mole-rat fibroblasts. Biochemical and Biophysical Research Communications, 528(1 Jul), 146–153. https://doi.org/10.1016/j.bbrc.2020.05.083.
Yu, H., Lin, L., Zhang, Z., Zhang, H., & Hu, H. (2020). Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduction and Targeted Therapy, 5(1 Sep), 209 https://doi.org/10.1038/s41392-020-00312-6.
Arneth, B. (2019). Tumor microenvironment. Medicina (B. Aires), 56(1 Dec), 15 https://doi.org/10.3390/medicina56010015.
Jin, M.-Z., & Jin, W.-L. (2020). The updated landscape of tumor microenvironment and drug repurposing. Signal Transduction and Targeted Therapy, 5(1 Aug), 166 https://doi.org/10.1038/s41392-020-00280-x.
Jiang, Y., Wang, C., & Zhou, S. (2020). Targeting tumor microenvironment in ovarian cancer: Premise and promise. Biochimica et Biophysica Acta - Reviews on Cancer, 1873(2 Apr), 188361. https://doi.org/10.1016/j.bbcan.2020.188361.
Tuccitto, A., et al. (2019). Immunosuppressive circuits in tumor microenvironment and their influence on cancer treatment efficacy. Virchows Archiv, 474(4 Apr), 407–420. https://doi.org/10.1007/s00428-018-2477-z.
Yarchoan, M., Hopkins, A., & Jaffee, E. M. (2017). Tumor mutational burden and response rate to PD-1 inhibition. The New England Journal of Medicine, 377(25 Dec), 2500–2501. https://doi.org/10.1056/NEJMc1713444.
Yang, L., Li, A., Lei, Q., & Zhang, Y. (2019). Tumor-intrinsic signaling pathways: key roles in the regulation of the immunosuppressive tumor microenvironment. Journal of Hematology & Oncology, 12(1 Dec), 125 https://doi.org/10.1186/s13045-019-0804-8.
Lu, C., et al. (2019). Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Molecular Cancer, 18(1 Dec), 130 https://doi.org/10.1186/s12943-019-1047-6.
Jin, Z., Sun, X., Wang, Y., Zhou, C., Yang, H. & Zhou, S. (2022) Regulation of autophagy fires up the cold tumor microenvironment to improve cancer immunotherapy. Frontiers in Immunology, 13, Oct, https://doi.org/10.3389/fimmu.2022.1018903.
Liu, Y., et al. (2023). Advances in immunotherapy for triple-negative breast cancer. Molecular Cancer, 22(1 Sep), 145. https://doi.org/10.1186/s12943-023-01850-7.
Zhang, Y., et al. (2022). Eliciting an immunostimulatory tumor microenvironment to enhance the antitumor efficacy by targeted cancer immunotherapy. Advances in Therapy, 5(9 Sep), 2200070. https://doi.org/10.1002/adtp.202200070.
Yin, S., Jin, W., Qiu, Y., Fu, L., Wang, T., & Yu, H. (2022). Solamargine induces hepatocellular carcinoma cell apoptosis and autophagy via inhibiting LIF/miR-192-5p/CYR61/Akt signaling pathways and eliciting immunostimulatory tumor microenvironment. Journal of Hematology & Oncology, 15(1 Dec), 32 https://doi.org/10.1186/s13045-022-01248-w.
Koontongkaew, S. (2013). The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. Journal of Cancer, 4(1), 66–83. https://doi.org/10.7150/jca.5112.
Sangaletti, S., Chiodoni, C., Tripodo, C., & Colombo, M. P. (2017). Common extracellular matrix regulation of myeloid cell activity in the bone marrow and tumor microenvironments. Cancer Immunology Immunotherapy, 66(8 Aug), 1059–1067. https://doi.org/10.1007/s00262-017-2014-y.
Kamińska, K., et al. (2015). The role of the cell-cell interactions in cancer progression. Journal of Cellular and Molecular Medicine, 19(2 Feb), 283–296. https://doi.org/10.1111/jcmm.12408.
Nishida-Aoki, N., & Gujral, T. S. (2019). Emerging approaches to study cell-cell interactions in tumor microenvironment. Oncotarget, 10(7 Jan), 785–797. https://doi.org/10.18632/oncotarget.26585.
Salazar, N., & Zabel, B. A. (2019) Support of tumor endothelial cells by chemokine receptors. Frontiers in Immunology, 10, Feb., https://doi.org/10.3389/fimmu.2019.00147.
Dudley, A. C. (2012). Tumor endothelial cells. Cold Spring Harbor Perspectives in Medicine, 2(3 Mar), a006536–a006536. https://doi.org/10.1101/cshperspect.a006536.
Lee, S. W., Kwak, H. S., Kang, M.-H., Park, Y.-Y., & Jeong, G. S. (2018). Fibroblast-associated tumour microenvironment induces vascular structure-networked tumouroid. Science Reports, 8(1 Feb), 2365 https://doi.org/10.1038/s41598-018-20886-0.
Kalluri, R. (2016). The biology and function of fibroblasts in cancer. Nature Reviews Cancer, 16(9 Sep), 582–598. https://doi.org/10.1038/nrc.2016.73.
Nurmik, M., Ullmann, P., Rodriguez, F., Haan, S., & Letellier, E. (2020). In search of definitions: cancer‐associated fibroblasts and their markers. International Journal of Cancer, 146(4 Feb), 895–905. https://doi.org/10.1002/ijc.32193.
Nishishita, R. et al. (2018) Expression of cancer-associated fibroblast markers in advanced colorectal cancer. Oncology Letters., Feb., https://doi.org/10.3892/ol.2018.8097.
Gok Yavuz, B., et al. (2019). Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1+ TAMs. Science Reports, 9(1 Feb), 3172 https://doi.org/10.1038/s41598-019-39553-z.
Wang, F., Sun, W., Zhang, J., & Fan, Y. (2019) Cancer‑associated fibroblast regulation of tumor neo‑angiogenesis as a therapeutic target in cancer (Review), Oncology Letters., Jan., https://doi.org/10.3892/ol.2019.9973.
Chen, D., Zhang, X., Li, Z., & Zhu, B. (2021). Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics, 11(3), 1016–1030. https://doi.org/10.7150/thno.51777.
Noy, R., & Pollard, J. W. (2014). Tumor-associated macrophages: from mechanisms to therapy. Immunity, 41(1 Jul), 49–61. https://doi.org/10.1016/j.immuni.2014.06.010.
Yunna, C., Mengru, H., Lei, W., & Weidong, C. (2020). Macrophage M1/M2 polarization. European Journal of Pharmacology, 877(Jun), 173090. https://doi.org/10.1016/j.ejphar.2020.173090.
Wang, F., et al. (2018). Interferon gamma induces reversible metabolic reprogramming of M1 macrophages to sustain cell viability and pro-inflammatory activity. EBioMedicine, 30(Apr), 303–316. https://doi.org/10.1016/j.ebiom.2018.02.009.
Wang, X., et al. (2022). Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Signal Transduction and Targeted Therapy, 7(1 Mar), 74 https://doi.org/10.1038/s41392-022-00894-3.
Chen, S., et al. (2023). Macrophages in immunoregulation and therapeutics. Signal Transduction and Targeted Therapy, 8(1 May), 207 https://doi.org/10.1038/s41392-023-01452-1.
Karakasheva, T. A., et al. (2018). IL-6 mediates cross-talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Research, 78(17 Sep), 4957–4970. https://doi.org/10.1158/0008-5472.CAN-17-2268.
Wei, C., et al. (2019). Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Molecular Cancer, 18(1 Dec), 64 https://doi.org/10.1186/s12943-019-0976-4.
Lin, Y., Xu, J., & Lan, H. (2019). Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. Journal of Hematology & Oncology, 12(1 Dec), 76 https://doi.org/10.1186/s13045-019-0760-3.
Kowal, J., Kornete, M., & Joyce, J. A. (2019). Re-education of macrophages as a therapeutic strategy in cancer. Immunotherapy, 11(8 Jun), 677–689. https://doi.org/10.2217/imt-2018-0156.
Xu, S., et al. (2019). The role of collagen in cancer: from bench to bedside. Journal of Translational Medicine, 17(1 Dec), 309 https://doi.org/10.1186/s12967-019-2058-1.
Huang, J., et al. (2021). Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduction and Targeted Therapy, 6(1 Apr), 153 https://doi.org/10.1038/s41392-021-00544-0.
Fang, T., et al. (2018). Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nature Communications, 9(1 Jan), 191 https://doi.org/10.1038/s41467-017-02583-0.
Javadian, M., et al. (2019). The role of microRNAs regulating the expression of matrix metalloproteinases (MMPs) in breast cancer development, progression, and metastasis. Journal of Cellular Physiology, 234(5 May), 5399–5412. https://doi.org/10.1002/jcp.27445.
Zhou, J. et al. (2023) The crosstalk between anoikis and epithelial-mesenchymal transition and their synergistic roles in predicting prognosis in colon adenocarcinoma. Frontiers in Oncology, 13, Jun, https://doi.org/10.3389/fonc.2023.1184215.
Bigagli, E., Cinci, L., D’Ambrosio, M., & Luceri, C. (2019). Transcriptomic characterization, chemosensitivity and regulatory effects of exosomes in spontaneous EMT/MET transitions of breast. Cancer Cells, Cancer Genomics - Proteomics, 16(3 Apr), 163–173. https://doi.org/10.21873/cgp.20122.
Kozlova, N., Grossman, J. E., Iwanicki, M. P., & Muranen, T. (2020). The interplay of the extracellular matrix and stromal cells as a drug target in stroma-rich cancers. Trends in Pharmacological Sciences, 41(3 Mar), 183–198. https://doi.org/10.1016/j.tips.2020.01.001.
Puré, E., & Lo, A. (2016). Can targeting stroma pave the way to enhanced antitumor immunity and immunotherapy of solid tumors? Cancer Immunology Research, 4(4 Apr), 269–278. https://doi.org/10.1158/2326-6066.CIR-16-0011.
Gao, Z. et al. (2018) Anoikis‑resistant human osteosarcoma cells display significant angiogenesis by activating the Src kinase‑mediated MAPK pathway. Oncology Reports, Oct., https://doi.org/10.3892/or.2018.6827.
Zhu, L., McManus, M. M., & Hughes, D. P. M. (2013) Understanding the Biology of bone sarcoma from early initiating events through late events in metastasis and disease progression. Frontiers in Oncology, 3, https://doi.org/10.3389/fonc.2013.00230.
Chang, C.-C., et al. (2013). CCN2 inhibits lung cancer metastasis through promoting DAPK-dependent anoikis and inducing EGFR degradation. Cell Death Differ, 20(3 Mar), 443–455. https://doi.org/10.1038/cdd.2012.136.
Lee, Y.-C., et al. (2013). Targeting constitutively activated β1 integrins inhibits prostate cancer metastasis. Molecular Cancer Research, 11(4 Apr), 405–417. https://doi.org/10.1158/1541-7786.MCR-12-0551.
Maycotte, P., Jones, K. L., Goodall, M. L., Thorburn, J., & Thorburn, A. (2015). Autophagy supports breast cancer stem cell maintenance by regulating IL6 secretion. Molecular Cancer Research, 13(4), 651–658. https://doi.org/10.1158/1541-7786.MCR-14-0487.
Luo, X., et al. (2021). The functions of autophagy at the tumour‐immune interface. Journal of Cellular and Molecular Medicine, 25(5 Mar), 2333–2341. https://doi.org/10.1111/jcmm.16331.
Chang, H., & Zou, Z. (2020). Targeting autophagy to overcome drug resistance: further developments. Journal of Hematology & Oncology, 13(1 Dec), 159. https://doi.org/10.1186/s13045-020-01000-2.
Wei, J., et al. (2016). Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nature Immunology, 17(3 Mar), 277–285. https://doi.org/10.1038/ni.3365.
Jena, B. C., Rout, L., Dey, A., & Mandal, M. (2021). Active autophagy in cancer‐associated fibroblasts: Recent advances in understanding the novel mechanism of tumor progression and therapeutic response. Journal of Cellular Physiology, 236(11 Nov), 7887–7902. https://doi.org/10.1002/jcp.30419.
Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K. J., & Werb, Z. (2020). Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nature Communications, 11(1 Oct), 5120. https://doi.org/10.1038/s41467-020-18794-x.
Ziegler, P. K., et al. (2018). Mitophagy in intestinal epithelial cells triggers adaptive immunity during tumorigenesis. Cell, 174(1 Jun), 88–101.e16. https://doi.org/10.1016/j.cell.2018.05.028.
Zhang, J., et al. (2019). Autophagy in regulatory T cells: a double-edged sword in disease settings. Molecular Immunology, 109(May), 43–50. https://doi.org/10.1016/j.molimm.2019.02.004.
Jacquin, E., & Apetoh, L. (2018) Cell-intrinsic roles for autophagy in modulating CD4 T cell functions. Frontiers in Immunology, 9, May, https://doi.org/10.3389/fimmu.2018.01023.
Xia, H., Green, D. R., & Zou, W. (2021). Autophagy in tumour immunity and therapy. Nature Reviews Cancer, 21(5 May), 281–297. https://doi.org/10.1038/s41568-021-00344-2.
Talmadge, J. E., & Gabrilovich, D. I. (2013). History of myeloid-derived suppressor cells. Nature Reviews Cancer, 13(10 Oct), 739–752. https://doi.org/10.1038/nrc3581.
Barsoum, I. B., Koti, M., Siemens, D. R., & Graham, C. H. (2014). Mechanisms of hypoxia-mediated immune escape in cancer. Cancer Research, 74(24 Dec), 7185–7190. https://doi.org/10.1158/0008-5472.CAN-14-2598.
Parker, K. H., Horn, L. A., & Ostrand-Rosenberg, S. (2016). High-mobility group box protein 1 promotes the survival of myeloid-derived suppressor cells by inducing autophagy. Journal of Leukocyte Biology, 100(3 Sep), 463–470. https://doi.org/10.1189/jlb.3HI0715-305R.
Kang, R., Livesey, K. M., Zeh, III, H. J., Loze, M. T., & Tang, D. (2010). HMGB1: a novel Beclin 1-binding protein active in autophagy. Autophagy, 6(8 Nov), 1209–1211. https://doi.org/10.4161/auto.6.8.13651.
Münz, C. (2016). Autophagy beyond intracellular MHC class II antigen presentation. Trends in Immunology, 37(11 Nov), 755–763. https://doi.org/10.1016/j.it.2016.08.017.
Jiang, G.-M., et al. (2019). The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Molecular Cancer, 18(1 Dec), 17 https://doi.org/10.1186/s12943-019-0944-z.
Lin, H., et al. (2013). Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll-like receptor 2-deficient mice. Hepatology, 57(1 Jan), 171–182. https://doi.org/10.1002/hep.25991.
Pereira, F. V., et al. (2018). Metformin exerts antitumor activity via induction of multiple death pathways in tumor cells and activation of a protective immune response. Oncotarget, 9(40 May), 25808–25825. https://doi.org/10.18632/oncotarget.25380.
Liu, J., Hong, M., Li, Y., Chen, D., Wu, Y., & Hu, Y. (2022) Programmed cell death tunes tumor immunity. Frontiers in Immunology, 13, Mar, https://doi.org/10.3389/fimmu.2022.847345.
Gregory, C. D., Ford, C. A., & Voss, J. J. L. P. Microenvironmental Effects of Cell Death in Malignant Disease, 2016, 51–88. https://doi.org/10.1007/978-3-319-39406-0_3.
Obeid, E., Nanda, R., Fu, Y.-X., & Olopade, O. I. (2013). The role of tumor-associated macrophages in breast cancer progression. International Journal of Oncology, 43(1 Jul), 5–12. https://doi.org/10.3892/ijo.2013.1938.
Ong, S.-M., et al. (2012). Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. European Journal of Immunology, 42(1 Jan), 89–100. https://doi.org/10.1002/eji.201141825.
Pantano, F., et al. (2013). The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. Journal of Cellular and Molecular Medicine, 17(11 Nov), 1415–1421. https://doi.org/10.1111/jcmm.12109.
Jung, K. Y., et al. (2015). Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. Journal of Pathology and Translational Medicine, 49(4 Jun), 318–324. https://doi.org/10.4132/jptm.2015.06.01.
Soki, F. N., et al. (2014). Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. Journal of Biological Chemistry, 289(35 Aug), 24560–24572. https://doi.org/10.1074/jbc.M114.571620.
Ford, C. A., et al. (2015). Oncogenic properties of apoptotic tumor cells in aggressive B cell lymphoma. Current Biology, 25(5 Mar), 577–588. https://doi.org/10.1016/j.cub.2014.12.059.
Verma, A., Warner, S. L., Vankayalapati, H., Bearss, D. J., & Sharma, S. (2011). Targeting Axl and Mer kinases in cancer. Molecular Cancer Therapy, 10(10 Oct), 1763–1773. https://doi.org/10.1158/1535-7163.MCT-11-0116.
Bosurgi, L., et al. (2013). Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proceedings of the National Academy of Sciences of the United States of America, 110(32 Aug), 13091–13096. https://doi.org/10.1073/pnas.1302507110.
Stanford, J. C., et al. (2014). Efferocytosis produces a prometastatic landscape during postpartum mammary gland involution. Journal of Clinical Investigation, 124(11 Nov), 4737–4752. https://doi.org/10.1172/JCI76375.
Sendoel, A., & Hengartner, M. O. (2014). Apoptotic cell death under hypoxia. Physiology, 29(3 May), 168–176. https://doi.org/10.1152/physiol.00016.2013.
Sermeus, A., et al. (2012). Hypoxia-induced modulation of apoptosis and BCL-2 family proteins in different cancer cell types. PLoS One, 7(11 Nov), e47519. https://doi.org/10.1371/journal.pone.0047519.
Pimentel, J. M., Zhou, J.-Y., & Wu, G. S. (2023). The role of TRAIL in apoptosis and immunosurveillance in cancer. Cancers (Basel), 15(10 May), 2752. https://doi.org/10.3390/cancers15102752.
Yuan, X., Gajan, A., Chu, Q., Xiong, H., Wu, K., & Wu, G. S. (2018). Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer and Metastasis Reviews, 37(4 Dec), 733–748. https://doi.org/10.1007/s10555-018-9728-y.
Liguori, M., et al. (2106). Functional TRAIL receptors in monocytes and tumor-associated macrophages: a possible targeting pathway in the tumor microenvironment. Oncotarget, 7(27 Jul), 41662–41676. https://doi.org/10.18632/oncotarget.9340.
de Looff, M., de Jong, S., & Kruyt, F. A. E. (2019) Multiple interactions between cancer cells and the tumor microenvironment modulate TRAIL signaling: implications for TRAIL receptor targeted therapy. Frontiers in Immunology, 10, Jul., https://doi.org/10.3389/fimmu.2019.01530.
van Roosmalen, I. A. M., Quax, W. J., & Kruyt, F. A. E. (2014). Two death-inducing human TRAIL receptors to target in cancer: similar or distinct regulation and function? Biochemical Pharmacology, 91(4 Oct), 447–456. https://doi.org/10.1016/j.bcp.2014.08.010.
Lecoultre, M., Dutoit, V., & Walker, P. R. (2020). Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: a review. The Journal of Immunotherapy of Cancer, 8(2 Dec), e001408 https://doi.org/10.1136/jitc-2020-001408.
Schmeisser, K., & Parker, J. A. (2019). Pleiotropic effects of mTOR and autophagy during development and aging. Frontiers in Cell and Developmental Biology, 7, 192 https://doi.org/10.3389/fcell.2019.00192.
Yu, J. S. L., & Cui, W. (2016). Proliferation, survival and metabolism: the role of PI3K/AKT/ mTOR signalling in pluripotency and cell fate determination. Development, 143(17), 3050–3060. https://doi.org/10.1242/dev.137075.
Karimi Roshan, M., Soltani, A., Soleimani, A., Rezaie Kahkhaie, K., Afshari, A. R., & Soukhtanloo, M. (2019). Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie, 165(Oct), 229–234. https://doi.org/10.1016/J.BIOCHI.2019.08.003.
Pires, B. R. B., et al. (2017). NF-kappaB is involved in the regulation of EMT genes in breast cancer cells. PLoS One, 12(1 Jan), e0169622. https://doi.org/10.1371/journal.pone.0169622. [Online]. Available.
Verzella, D., et al. (2020). Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death & Disease, 11(3), 210 https://doi.org/10.1038/s41419-020-2399-y.
He, Z. J., Zhu, F. Y., Li, S. S., Zhong, L., Tan, H. Y., & Wang, K. (2017). Inhibiting ROS-NF-κB-dependent autophagy enhanced brazilin-induced apoptosis in head and neck squamous cell carcinoma. Food and Chemical Toxicology, 101(Mar), 55–66. https://doi.org/10.1016/J.FCT.2017.01.002.
Niu, T., Tian, Y., Wang, G., Guo, G., Tong, Y., & Shi, Y. (2020). Inhibition of ROS-NF-κB-dependent autophagy enhances Hypocrellin A united LED red light-induced apoptosis in squamous carcinoma A431 cells. Cell Signaling, 69(May), 109550 https://doi.org/10.1016/J.CELLSIG.2020.109550.
Han, J. H., et al. (2022). Snail acetylation by autophagy‐derived acetyl‐coenzyme A promotes invasion and metastasis of KRAS ‐ LKB1 co‐mutated lung cancer cells. Cancer Commun, 42(8 Aug), 716–749. https://doi.org/10.1002/cac2.12332.
Zhang, X., Wang, H., Yu, M., Ma, K., & Ning, L. (2022). Inhibition of autophagy by 3-methyladenine promotes migration and invasion of colon cancer cells through epithelial mesenchymal transformation. Translational Cancer Research, 11(8 Aug), 2834–2842. https://doi.org/10.21037/tcr-22-1736.
Wang, Y., et al. (2019). Autophagy inhibition specifically promotes epithelial-mesenchymal transition and invasion in RAS-mutated cancer cells. Autophagy, 15(5 May), 886–899. https://doi.org/10.1080/15548627.2019.1569912.
Lamark, T., Svenning, S., & Johansen, T. (2017). Regulation of selective autophagy: the p62/SQSTM1 paradigm. Essays in Biochemistry, 61(6 Dec), 609–624. https://doi.org/10.1042/EBC20170035.
Li, J., et al. (2013). Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial–mesenchymal transition. Carcinogenesis, 34(6 Jun), 1343–1351. https://doi.org/10.1093/carcin/bgt063.
Song, J., et al. (2011). Autophagy in hypoxia protects cancer cells against apoptosis induced by nutrient deprivation through a beclin1-dependent way in hepatocellular carcinoma. Journal of Cellular Biochemistry, 112(11 Nov), 3406–3420. https://doi.org/10.1002/jcb.23274.
Chen, H.-T., et al. (2019). Crosstalk between autophagy and epithelial-mesenchymal transition and its application in cancer therapy. Molecular Cancer, 18(1 Dec), 101 https://doi.org/10.1186/s12943-019-1030-2.
Powan, P., Luanpitpong, S., He, X., Rojanasakul, Y., & Chanvorachote, P. (2017). Detachment-induced E-cadherin expression promotes 3D tumor spheroid formation but inhibits tumor formation and metastasis of lung cancer cells. American Journal of Physiology, 313(5 Nov), C556–C566. https://doi.org/10.1152/ajpcell.00096.2017.
Signore, M., Ricci-Vitiani, L., & De Maria, R. (2013). Targeting apoptosis pathways in cancer stem cells. Cancer Letters, 332(2 May), 374–382. https://doi.org/10.1016/j.canlet.2011.01.013.
Vitale, I., Manic, G., Dandrea, V., & De Maria, R. (2015). Role of autophagy in the maintenance and function of cancer stem cells. The International Journal of Developmental Biology, 59(1-2–3), 95–108. https://doi.org/10.1387/ijdb.150082iv.
Russell, R. C., & Guan, K. (2022). The multifaceted role of autophagy in cancer. EMBO Journal, 41(Jul), 13 https://doi.org/10.15252/embj.2021110031.
Qiang, L., et al. (2014). Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proceedings of the National Academy of Sciences of the United States of America, 111(25 Jun), 9241–9246. https://doi.org/10.1073/pnas.1322913111.
Tanabe, S., Quader, S., Cabral, H., & Ono, R. (2020) Interplay of EMT and CSC in cancer and the potential therapeutic strategies. Frontiers in Pharmacology, 11, Jun., https://doi.org/10.3389/fphar.2020.00904.
Leng, X., Huang, G., Li, S., Yao, M., Ding, J., & Ma, F. (2021). Correlation of breast cancer microcirculation construction with tumor stem cells (CSCs) and epithelial-mesenchymal transition (EMT) based on contrast-enhanced ultrasound (CEUS). PLoS One, 16(12 Dec), e0261138. https://doi.org/10.1371/journal.pone.0261138.
Dong, B., Li, S., Zhu, S., Yi, M., Luo, S., & Wu, K. (2021). MiRNA-mediated EMT and CSCs in cancer chemoresistance. Experimental Hematology & Oncology, 10(1 Dec), 12. https://doi.org/10.1186/s40164-021-00206-5.
Bertrand, M., et al. (2015). SQSTM1/p62 regulates the expression of junctional proteins through epithelial-mesenchymal transition factors. Cell Cycle, 14(3 Feb), 364–374. https://doi.org/10.4161/15384101.2014.987619.
Xu, L.-Z., et al. (2017). p62/SQSTM1 enhances breast cancer stem-like properties by stabilizing MYC mRNA. Oncogene, 36(3 Jan), 304–317. https://doi.org/10.1038/onc.2016.202.
Gong, C., et al. (2013). Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene, 32(18 May), 2261–2272. https://doi.org/10.1038/onc.2012.252.
Aguilar-Gallardo, C., Zamorano, M., Farias, J. G., & Quevedo, K. D. A. (2022). Understanding autophagy role in cancer stem cell development. Molecular Biology Reports, 49(7 Jul), 6741–6751. https://doi.org/10.1007/s11033-022-07299-z.
Nuñez-Olvera, S. I. et al. (2019) Autophagy machinery as a promising therapeutic target in endometrial cancer. Frontiers in Oncology, 9. Frontiers Media S.A., Nov. https://doi.org/10.3389/fonc.2019.01326.
Talesa, V. N., Ferri, I., Bellezza, G., Love, H. D., Sidoni, A., & Antognelli, C. (2017). Glyoxalase 2 is involved in human prostate cancer progression as part of a mechanism driven by PTEN/PI3K/AKT/mTOR signaling with involvement of PKM2 and ERα. Prostate, 77(2 Feb), 196–210. https://doi.org/10.1002/pros.23261.
Slomovitz, B. M., & Coleman, R. L. (2012). The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial. Cancer, AACR, 18(21 Nov), 5856–5864. https://doi.org/10.1158/1078-0432.CCR-12-0662.
Li, L., & Leung, P. S. (2014). Use of herbal medicines and natural products: an alternative approach to overcoming the apoptotic resistance of pancreatic cancer. The International Journal of Biochemistry & Cell Biology, 53(Aug), 224–236. https://doi.org/10.1016/j.biocel.2014.05.021.
Zeng, X., Yan, T., Schupp, J. E., Seo, Y., & Kinsella, T. J. (2007). DNA mismatch repair initiates 6-thioguanine–induced autophagy through p53 activation in human tumor cells. Clinical Cancer Research, 13(4 Feb), 1315–1321. https://doi.org/10.1158/1078-0432.CCR-06-1517.
Cherblanc, F. L., Davidson, R. W. M., Di Fruscia, P., Srimongkolpithak, N., & Fuchter, M. J. (2013). Perspectives on natural product epigenetic modulators in chemical biology and medicine. Natural Product Report, 30(5), 605. https://doi.org/10.1039/c3np20097c.
Kumari, N., Dwarakanath, B. S., Das, A., & Bhatt, A. N. (2016). Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biology, 37(9), 11553–11572. https://doi.org/10.1007/s13277-016-5098-7.
Kumari, N., Das, A., & Bhatt, A. N. (2020). Interleukin-6 confers radio-resistance by inducing Akt-mediated glycolysis and reducing mitochondrial damage in cells. Journal of Biochemistry, 167(3 Mar), 303–314. https://doi.org/10.1093/jb/mvz091.
Mylavarapu, S., Das, A., & Roy, M. (2018). Role of BRCA mutations in the modulation of response to platinum therapy. Frontiers in Oncology, 8(Feb), 16 https://doi.org/10.3389/fonc.2018.00016.
Luo, M.-L., Huang, W., Zhu, H.-P., Peng, C., Zhao, Q., & Han, B. (2022). Advances in indole-containing alkaloids as potential anticancer agents by regulating autophagy. Biomedicine & Pharmacotherapy, 149, 112827 https://doi.org/10.1016/j.biopha.2022.112827.
Wang, J., et al. (2021). Sanguinarine impairs lysosomal function and induces ROS-dependent mitophagy and apoptosis in human hepatocellular carcinoma cells. Archieves of Pharmacal Research, 44(11), 1025–1036. https://doi.org/10.1007/s12272-021-01356-0.
Zhai, K., Siddiqui, M., Abdellatif, B., Liskova, A., Kubatka, P., & Büsselberg, D. (2021). Natural compounds in glioblastoma therapy: preclinical insights, mechanistic pathways, and outlook. Cancers (Basel), 13, 10 https://doi.org/10.3390/cancers13102317.
Kumar, S., & Das, A. (2023) A cocktail of natural compounds holds promise for new immunotherapeutic potential in head and neck cancer, Chinese Journal of Integrative Medicine, Apr., https://doi.org/10.1007/s11655-023-3694-0.
Sakamoto, S., Kypriyanau, N. (2021) Aspects of medicine, and undefined 2010, Targeting anoikis resistance in prostate cancer metastasis, Elsevier, Accessed: Apr. 27. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0098299710000026.
Keledjian, K., Garrison, J. B., & Kyprianou, N. (2005). Doxazosin inhibits human vascular endothelial cell adhesion, migration, and invasion. Journal of Cellular Biochemistry, 94(2 Feb), 374–388. https://doi.org/10.1002/jcb.20240.
Banach, A., Jiang, Y.-P., Roth, E., Kuscu, C., Cao, J., & Lin, R. Z. (2019). CEMIP upregulates BiP to promote breast cancer cell survival in hypoxia. Oncotarget, 10(42 Jul), 4307–4320. https://doi.org/10.18632/oncotarget.27036.
Liu, B., et al. (2022). CEMIP promotes extracellular matrix-detached prostate cancer cell survival by inhibiting ferroptosis. Cancer Science, 113(6 Jun), 2056–2070. https://doi.org/10.1111/cas.15356.
Souers, A. J., et al. (2013). ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature Medicine, 19(2), 202–208. https://doi.org/10.1038/nm.3048.
Edlich, F. (2018). BCL-2 proteins and apoptosis: recent insights and unknowns. Biochemical and Biophysical Research Communications, 500(1), 26–34. https://doi.org/10.1016/j.bbrc.2017.06.190.
Yadav, A., Kumar, B., Yu, J.-G., Old, M., Teknos, T. N., & Kumar, P. (2015). Tumor-associated endothelial cells promote tumor metastasis by chaperoning circulating tumor cells and protecting them from anoikis. PLoS One, 10(10 Oct), e0141602 https://doi.org/10.1371/journal.pone.0141602.
Saleem, S. (2021). Apoptosis, autophagy, necrosis and their multi galore crosstalk in neurodegeneration. Neuroscience, 469(Aug), 162–174. https://doi.org/10.1016/j.neuroscience.2021.06.023.
Ojha, R., Ishaq, M., & Singh, S. (2015). Caspase-mediated crosstalk between autophagy and apoptosis: mutual adjustment or matter of dominance. Journal of Cancer Research and Therapeutics, 11(3), 514 https://doi.org/10.4103/0973-1482.163695.
Wolf, P., Schoeniger, A., & Edlich, F. (2022). Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane. Biochimica et Biophysica Acta - Molecular Cell Research, 1869(10), 119317 https://doi.org/10.1016/j.bbamcr.2022.119317.
Kale, J., et al. (2018). Phosphorylation switches Bax from promoting to inhibiting apoptosis thereby increasing drug resistance. EMBO Reports, 19(9), e45235 https://doi.org/10.15252/embr.201745235.
Dong, W., et al. (2012). IKKα contributes to UVB-induced VEGF expression by regulating AP-1 transactivation. Nucleic Acids Research, 40(7 Apr), 2940–2955. https://doi.org/10.1093/nar/gkr1216.
Xu, X., Zhang, C., Xu, H., Wu, L., Hu, M., & Song, L. (2020) Autophagic feedback-mediated degradation of IKKα requires CHK1/p300/CBP-dependent acetylation of p53. Journal of Cell Science, Jan, https://doi.org/10.1242/jcs.246868.
Tan, Q., et al. (2020). Selective degradation of IKKα by autophagy is essential for arsenite-induced cancer cell apoptosis. Cell Death & Disease, 11(4 Apr), 222 https://doi.org/10.1038/s41419-020-2420-5.
Yuan, J., et al. (2021). MiRNA-223-3p affects mantle cell lymphoma development by regulating the CHUK/NF-ƘB2 signaling pathway. OncoTargets and Therapy, 14, 1553–1564. https://doi.org/10.2147/OTT.S283486.
Rahal, R., et al. (2014). Pharmacological and genomic profiling identifies NF-κB-targeted treatment strategies for mantle cell lymphoma. Nature Medicine, 20(1 Jan), 87–92. https://doi.org/10.1038/nm.3435.
Cao, Y. (2011) Mechanisms of the apoptosis induced by CD176 antibody in human leukemic cells. International Journal of Oncology, Mar., https://doi.org/10.3892/ijo.2011.992.
Li, X., & Hu, Y. (2021). Attribution of NF-κB Activity to CHUK/IKKα-Involved Carcinogenesis. Cancers (Basel), 13(6 Mar), 1411 https://doi.org/10.3390/cancers13061411.
Chen, N., & Debnath, J. (2013). IκB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway. Autophagy, 9(8 Aug), 1214–1227. https://doi.org/10.4161/auto.24870.
Gradzka, S., et al. (2018). Inhibitor of apoptosis proteins are required for effective fusion of autophagosomes with lysosomes. Cell Death & Disease, 9(5 May), 529 https://doi.org/10.1038/s41419-018-0508-y.
Yin, Z., Pascual, C., & Klionsky, D. (2016). Autophagy: machinery and regulation. Microbial Cell, 3(12 Dec), 588–596. https://doi.org/10.15698/mic2016.12.546.
Berthelet, J., & Dubrez, L. (2013). Regulation of apoptosis by inhibitors of apoptosis (IAPs). Cells, 2(1 Mar), 163–187. https://doi.org/10.3390/cells2010163.
Frazzi, R. (2021). BIRC3 and BIRC5: multi‐faceted inhibitors in cancer. Cell Bioscience, 11(1), 8 https://doi.org/10.1186/s13578-020-00521-0.
Cetraro, P., Plaza-Diaz, J., Mackenzie, A., & Abadía-Molina, F. (2022). A review of the current impact of inhibitors of apoptosis proteins and their repression in cancer. Cancers (Basel), 14(7), 1–25. https://doi.org/10.3390/cancers14071671.
Dai, Y., & Grant, S. (2015). BCL2L11/Bim as a dual-agent regulating autophagy and apoptosis in drug resistance. Autophagy, 11(2 Feb), 416–418. https://doi.org/10.1080/15548627.2014.998892.
Delgado, M., & Tesfaigzi, Y. (2014). Is BMF central for anoikis and autophagy? Autophagy, 10(1 Jan), 168–169. https://doi.org/10.4161/auto.26759.
Zhi, Z., et al. (2022). Non-canonical phosphorylation of Bmf by p38 MAPK promotes its apoptotic activity in anoikis. Cell Death and Differentiation, 29(2 Feb), 323–336. https://doi.org/10.1038/s41418-021-00855-3.
Fan, Y.-X. et al. (2018) MicroRNA-125b inhibits cell proliferation and induces cell apoptosis in esophageal squamous cell carcinoma by targeting BMF. Oncology Reports, May, https://doi.org/10.3892/or.2018.6413.
Shalini, S., Dorstyn, L., Dawar, S., & Kumar, S. (2015). Old, new and emerging functions of caspases. Cell Death and Differentiation, 22(4 Apr), 526–539. https://doi.org/10.1038/cdd.2014.216.
Tiwari, M., et al. (2014). A nonapoptotic role for CASP2/caspase 2. Autophagy, 10(6 Jun), 1054–1070. https://doi.org/10.4161/auto.28528.
Pagliarini, V., et al. (2012). Proteolysis of Ambra1 during apoptosis has a role in the inhibition of the autophagic pro-survival response. Cell Death and Differentiation, 19(9 Sep), 1495–1504. https://doi.org/10.1038/cdd.2012.27.
Betin, V. M. S., & Lane, J. D. (2009). Atg4D at the interface between autophagy and apoptosis. Autophagy, 5(7 Oct), 1057–1059. https://doi.org/10.4161/auto.5.7.9684.
Bouchier-Hayes, L., & Green, D. R. (2012). Caspase-2: the orphan caspase. Cell Death and Differentiation, 19(1 Jan), 51–57. https://doi.org/10.1038/cdd.2011.157.
Mandal, R., Barrón, J. C., Kostova, I., Becker, S., & Strebhardt, K. (2020). Caspase-8: the double-edged sword. Biochimica et Biophysica Acta - Reviews on Cancer, 1873(2 Apr), 188357 https://doi.org/10.1016/j.bbcan.2020.188357.
Kumar, M., Irungbam, K., & Kataria, M. (2018). Depletion of membrane cholesterol compromised caspase-8 imparts in autophagy induction and inhibition of cell migration in cancer cells. Cancer Cell International, 18(1 Dec), 23. https://doi.org/10.1186/s12935-018-0520-4.
Norman, J. M., Cohen, G. M., & Bampton, E. T. W. (2010). The in vitro cleavage of the hAtg proteins by cell death proteases. Autophagy, 6(8 Nov), 1042–1056. https://doi.org/10.4161/auto.6.8.13337.
Elgendy, M., Sheridan, C., Brumatti, G., & Martin, S. J. (2011). Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Molecular Cell, 42(1 Apr), 23–35. https://doi.org/10.1016/j.molcel.2011.02.009.
Orrenius, S., Gogvadze, V., & Zhivotovsky, B. (2015). Calcium and mitochondria in the regulation of cell death. Biochemical and Biophysical Research Communications, 460(1), 72–81. https://doi.org/10.1016/j.bbrc.2015.01.137.
Ghaemi, S., Arefian, E., Rezazadeh Valojerdi, R., Soleimani, M., Moradimotlagh, A., & Jamshidi Adegani, F. (2020). Inhibiting the expression of anti-apoptotic genes BCL2L1 and MCL1, and apoptosis induction in glioblastoma cells by microRNA-342. Biomedicine & Pharmacotherapy, 121(Jan), 109641 https://doi.org/10.1016/j.biopha.2019.109641.
Chunhacha, P., Pongrakhananon, V., Rojanasakul, Y., & Chanvorachote, P. (2012). Caveolin-1 regulates Mcl-1 stability and anoikis in lung carcinoma cells. American Journal of Physiology, 302(9 May), C1284–C1292. https://doi.org/10.1152/ajpcell.00318.2011.
Criollo, A., et al. (2010). The IKK complex contributes to the induction of autophagy. EMBO Journal, 29(3 Feb), 619–631. https://doi.org/10.1038/emboj.2009.364.
Zada, S., et al. (2021). Cross talk between autophagy and oncogenic signaling pathways and implications for cancer therapy. Biochimica et Biophysica Acta - Reviews on Cancer, 1876(1 Aug), 188565 https://doi.org/10.1016/j.bbcan.2021.188565.
Zhang, P., et al. (2016). Tetraiodothyroacetic acid and transthyretin silencing inhibit pro-metastatic effect of L-thyroxin in anoikis-resistant prostate cancer cells through regulation of MAPK/ERK pathway. Experimental Cell Research, 347(2 Oct), 350–359. https://doi.org/10.1016/j.yexcr.2016.08.019.
Dower, C. M., Wills, C. A., Frisch, S. M., & Wang, H. G. (2018). Mechanisms and context underlying the role of autophagy in cancer metastasis. Autophagy, 14(7), 1110–1128. https://doi.org/10.1080/15548627.2018.1450020.
Das Gupta, D., et al. (2021). Evaluation of antioxidant, anti-inflammatory and anticancer activities of diosgenin enriched Paris polyphylla rhizome extract of Indian Himalayan landraces. Journal of Ethnopharmacology, 270(Apr), 113842. https://doi.org/10.1016/j.jep.2021.113842.
Redza-Dutordoir, M., & Averill-Bates, D. A. (2016). Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta - Molecular Cell Research, 1863(12 Dec), 2977–2992. https://doi.org/10.1016/j.bbamcr.2016.09.012.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and analysis were performed by the corresponding author, A.D. The first draft of the manuscript was written by S.G. and P.C. commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gulia, S., Chandra, P. & Das, A. The Prognosis of Cancer Depends on the Interplay of Autophagy, Apoptosis, and Anoikis within the Tumor Microenvironment. Cell Biochem Biophys 81, 621–658 (2023). https://doi.org/10.1007/s12013-023-01179-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-023-01179-4