Abstract
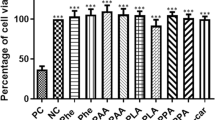
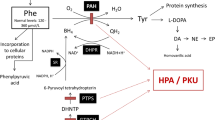
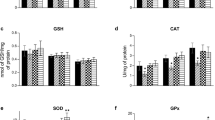
Phenylketonuria (PKU) is an inherited metabolic disorder caused by deficiency of phenylalanine hydroxylase which leads to accumulation of phenylalanine and its metabolites in tissues of patients with severe neurological involvement. Recently, many studies in animal models or patients have reported the role of oxidative stress in PKU. In the present work we studied the effect of lipoic acid against oxidative stress in rat brain provoked by an animal model of hyperphenylalaninemia (HPA), induced by repetitive injections of phenylalanine and α-methylphenylalanine (a phenylalanine hydroxylase inhibitor) for 7 days, on some oxidative stress parameters. Lipoic acid prevented alterations on catalase (CAT) and superoxide dismutase (SOD), and the oxidative damage of lipids, proteins, and DNA observed in HPA rats. In addition, lipoic acid diminished reactive species generation compared to HPA group which was positively correlated to SOD/CAT ratio. We also observed that in vitro Phe inhibited CAT activity while phenyllactic and phenylacetic acids stimulated superoxide dismutase activity. These results demonstrate the efficacy of lipoic acid to prevent oxidative stress induced by HPA model in rats. The possible benefits of lipoic acid administration to PKU patients should be considered.




Similar content being viewed by others
References
Acosta PB, Yannicelli S, Marriage B, Steiner R, Gaffield B, Arnold G, Lewis V, Cho S, Berstein L, Parton P, Leslie N, Korson M (1999) Protein status of infants with phenylketonuria undergoing nutrition management. J Am Coll Nutr 18:102–107
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Dyer CA (1999) Pathophysiology of phenylketonuria. Ment Retard Dev Disabil Res Rev 5:104–112
Ercal N, Aykin-Burns N, Gurer-Orhan H, McDonald JD (2002) Oxidative stress in a phenylketonuria animal model. Free Radic Biol Med 32:906–911
Fernandes CG, Leipnitz G, Seminotti B, Amaral AU, Zanatta A, Vargas CR, Dutra Filho CS, Wajner M (2010) Experimental evidence that phenylalanine provokes oxidative stress in hippocampus and cerebral cortex of developing rats. Cell Mol Neurobiol 30:317–326
Garrett NE, Malcangio M, Dewhurst M, Tomlinson DR (1997) α-Lipoic acid corrects neuropeptide deficits in diabetic rats via induction of trophic support. Neurosci Lett 222:191–194
Gassió R, Artuch R, Vilaseca MA, Fusté E, Colome R, Campistol J (2008) Cognitive functions and the antioxidant system in phenylketonuric patients. Neuropsychology 22(4):426–431
Hagen MEK, Pederzolli CD, Sgaravatti AM, Bridi R, Wajner M, Wannmacher CM, Wyse AT, Dutra-Filho CS (2002) Experimental hyperphenylalaninemia provokes oxidative stress in rat brain. Biochim Biophys Acta 1586:344–352
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, New York
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br J Pharmacol 142:231–255
Kayaalp E, Treacy E, Waters PJ, Byck S, Nowacki P, Scriver CR (1997) Human phenylalanine hydroxylase mutations and hyperphenylalaninemia phenotypes: a meta-analysis of genotypephenotype correlations. Am J Hum Genet 61:1309–1317
Kim MY, Kim EJ, Kim Y, Choi C, Lee B (2011) Effects of α-lipoic acid and l-carnosine supplementation on antioxidant activities and lipid profiles in rats. Nutr Res Pract 5(5):421–428
Lebel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Rés Toxicol 5:227–231
Li Y, Zhao Y, Yu W, Jiang S (2004) Scavenging ability on ROS of alpha-lipoic acid (ALA). Food Chem 84:563–567
Lissi E, Caceres T, Videla LA (1986) Visible chemiluminescence from rat brain homogenates undergoing autoxidation. I. Effect of additives and products accumulation. Free Radic Biol Med 2:63–69
Llesuy SF, Milei J, Molina H, Boveris A, Milei S (1985) Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4′-epiadriamycin in mice. Tumori 71:241–249
Lowry OH, Rosebrough NJ, Lewis-Farr A, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Marklund SL (1985) Pyrogallol autoxidation. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 243–247
Mazzola PN, Terra M, Rosa AP, Mescka CP, Moraes TB, Piccoli B, Jacques CE, Dalazen GR, Cortes MX, Coelho JC, Dutra-Filho CS (2011) Regular exercise prevents oxidative stress in the brain of hyperphenylalaninemic rats. Metab Brain Dis 26:291–297
Moraes TB, Zanin F, da Rosa A, de Oliveira A, Coelho J, Petrillo F, Wajner M, Dutra-Filho CS (2010) Lipoic acid prevents oxidative stress in vitro and in vivo by an acute hyperphenylalaninemia chemically-induced in rat brain. J Neurol Sci 292:89–95
Nistico G, Ciriolo MR, Fiskin K, Iannone M, De Martino A, Rotilio G (1992) NGF restores decrease in catalase activity and increases superoxide dismutase and glutathione peroxidase activity in the brain of aged rats. Free Radic Biol Med 12:177–181
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Packer L, Eric HW, Hans JT (1995) Alpha lipoic acid as a biological antioxidant. Free Rad Biol Med 19:227–250
Packer L, Tritschler HJ, Wessel K (1997) Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Radic Biol Med 22:359–378
Pederzolli CD, Rosa AP, Oliveira AS, Coelho JG, Becker DL, Dalazen GR, Moraes TB, Dutra-Filho CS (2010) Neuroprotective role of lipoic acid against acute toxicity of N-acetylaspartic acid. Mol Cell Biochem 344:231–239
Perera J, Tan JH, Jeevathayaparan S, Chakravarthi S, Haleagrahara N (2011) Neuroprotective effects of alpha lipoic acid on haloperidol-induced oxidative stress in the rat brain. Cell Biosci 1:1–12
Podda M, Rallis M, Cuber MG, Packer L, Maibachl H (1996) Kinetic study of cutaneous and subcutaneous distribution following topical application of lipoic acid onto hairless mice. Biochem Pharmacol 52:627–633
Potocnik U, Widhalm K (1994) Long-term follow-up of children with classical phenylketonuria after diet discontinuation: a review. J Am Coll Nutr 3:232–236
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Ribas GS, Sitta A, Wajner M, Vargas CR (2011) Oxidative stress in phenylketonuria: What is the evidence? Cell Mol Neurobiol 31(5):653–662
Ribas GS, Biancini GB, Mescka CP, Wayhs CY, Sitta A, Wajner M, Vargas CR (2012) Oxidative stress parameters in urine from patients with disorders of propionate metabolism: a beneficial effect of l-carnitine supplementation. Cell Mol Neurobiol 32:77–82
Ristow M, Zarse K (2010) How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 45:410–418
Rocha JC, Martins MJ (2012) Oxidative stress in Phenylketonuria: future directions. J Inherit Metab Dis 35:381–398
Samuel S, Kathirvel R, Jayavelu T, Chinnakkannu P (2005) Protein oxidativee damage in arsenic induced rat brain: influence of dl-α-lipoic acid. Toxicol Lett 155:27–34
Sanayama Y, Nagasaka H, Takayanagi M, Ohura T, Sakamoto O, Ito T, Ishige-Wada M, Usui H, Yoshino M, Ohtake A, Yorifuji T, Tsukahara H, Hirayama S, Miida T, Fukui M, Okano Y (2011) Experimental evidence that phenylalanine is strongly associated to oxidative stress in adolescents and adults with phenylketonuria. Mol Genet Metab 103(3):220–225
Scriver CR, Kaufman S (2001) Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1667–1724
Sierra C, Vilaseca MA, Moyano D, Brandi N, Campistol J, Lambruschini N, Cambra FJ, Deulofeu R, Mira A (1998) Antioxidant status in hyperphenylalaninemia. Clin Chim Acta 276:1–9
Sirtori LR, Dutra-Filho CS, Fitarelli D, Sitta A, Haeser A, Barschak AG, Wajner M, Coelho DM, Llesuy S, Bello′-Klein A, Giugliani R, Deon M, Vargas CR (2005) Oxidative stress in patients with phenylketonuria. Biochim Biophys Acta 1740:68–73
Sitta A, Barschak AG, Deon M, Terroso T, Pires R, Giugliani R, Dutra-Filho CS, Wajner M, Vargas CR (2006) Investigation of oxidative stress parameters in treated phenylketonuric patients. Metab Brain Dis 4:287–296
Sitta A, Manfredini V, Biasi L, Treme′a R, Schwartz IV, Wajner M, Vargas CR (2009a) Evidence that DNA damage is associated to phenylalanine blood levels in leukocytes from phenylketonuric patients. Mutat Res 679:13–16
Sitta A, Barschak AG, Deon M, Barden AT, Biancini GB, Vargas PR, de Souza CF, Netto C, Wajner M, Vargas CR (2009b) Effect of short- and long-term exposition to high phenylalanine blood levels on oxidative damage in phenylketonuric patients. Int J Dev Neurosci 27:243–247
Sitta A, Vanzin CS, Biancini GB, Manfredini V, de Oliveira AB, Wayhs CA, Ribas GO, Giugliani L, Schwartz IV, Bohrer D, Garcia SC, Wajner M, Vargas CR (2011) Evidence that l-carnitine and selenium supplementation reduces oxidative stress in phenylketonuric patients. Cell Mol Neurobiol 31:429–436
Stadtman ER (1990) Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med 9:315–325
Stadtman ER, Levine RL (2003) Free-radical mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25:207–218
van Backel MME, Printzen G, Wermuth B, Wiesmann UN (2000) Antioxidant and thyroid hormone status in selenium-deficient phenylketonuric and hyperphenylalaninemic patients. Am J Clin Nutr 72:976–981
Vanzin CS, Biancini GB, Sitta A, Wayhs CAY, Pereira YN, Rockenbach F, Garcia SC, Wyse ATS, Schwartz IVD, Wajner M, Vargas CR (2011) Experimental evidence of oxidative stress in plasma of homocystinuric patients: a possible role for homocysteine. Mol Genet Metab 104:112–117
Vargas CR, Wajner M, Sitta A (2011) Oxidative stress in phenylketonuric patients. Mol Genet Metab 104:S97–S99
Wajner M, Latini A, Wyse AT, Dutra-Filho CS (2004) The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis 27:427–448
Zhitkovich A, Costa M (1992) A simple, sensitive assay to detect DNA protein-crosslinks in intact cells and in vivo. Carcinogenesis 13:1485–1489
Acknowledgments
This work was supported by the research grants from Brazilian National Research Council (CNPq), Programa de Núcleos de Excelência (PRONEX), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), PROPESQ/UFRGS, and FINEP/Rede Instituto Brasileiro de Neurociência (IBN-Net) #01.06.0842-00.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moraes, T.B., Jacques, C.E.D., Rosa, A.P. et al. Role of Catalase and Superoxide Dismutase Activities on Oxidative Stress in the Brain of a Phenylketonuria Animal Model and the Effect of Lipoic Acid. Cell Mol Neurobiol 33, 253–260 (2013). https://doi.org/10.1007/s10571-012-9892-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-012-9892-5