Abstract
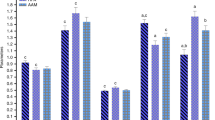
It is well established that the involvement of reactive species in the pathophysiology of several neurological diseases, including phenylketonuria (PKU), a metabolic genetic disorder biochemically characterized by elevated levels of phenylalanine (Phe). In previous studies, we verified that PKU patients (treated with a protein-restricted diet supplemented with a special formula not containing l-carnitine and selenium) presented high lipid and protein oxidative damage as well as a reduction of antioxidants when compared to the healthy individuals. Our goal in the present study was to evaluate the effect of Phe-restricted diet supplemented with l-carnitine and selenium, two well-known antioxidant compounds, on oxidative damage in PKU patients. We investigated various oxidative stress parameters in blood of 18 treated PKU patients before and after 6 months of supplementation with a special formula containing l-carnitine and selenium. It was verified that treatment with l-carnitine and selenium was capable of reverting the lipid peroxidation, measured by thiobarbituric acid-reactive species, and the protein oxidative damage, measured by sulfhydryl oxidation, to the levels of controls. Additionally, the reduced activity of glutathione peroxidase was normalized by the antioxidant supplementation. It was also verified a significant inverse correlation between lipid peroxidation and l-carnitine blood levels as well as a significant positive correlation between glutathione peroxidase activity and blood selenium concentration. In conclusion, our results suggest that supplementation of l-carnitine and selenium is important for PKU patients since it could help to correct the oxidative stress process which possibly contributes, at least in part, to the neurological symptoms found in phenylketonuric patients.



Similar content being viewed by others
References
Acosta PB (1996) Nutrition studies in treated infants and children with phenylketonuria: vitamins, minerals, trace elements. Eur J Pediatr 155:136–139
Adibhatla RM, Hatcher JF (2010) Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 12:125–169
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Artuch R, Vilaseca MA, Moreno J, Lambruschini N, Cambra FJ, Campistol J (1999) Decreased serum ubiquinone-10 concentration in phenylketonuria. Am J Clin Nutr 70:892–895
Artuch R, Colome C, Vilaseca MA, Sierra C, Cambra FJ, Lambruschini N, Campistol J (2001) Plasma phenylalanine is associated with decreased serum ubiquinone-10 concentrations in phenylketonuria. J Inherit Metab Dis 24:359–366
Artuch R, Colome C, Sierra C, Brandi N, Lambruschini N, Campistol J, Ugarte D, Vilaseca MA (2004) A longitudinal study of antioxidant status in phenylketonuric patients. Clin Biochem 37:198–203
Barschak AG, Sitta A, Deon M, Busanello EN, Coelho DM, Cipriani F, Dutra-Filho CS, Giugliani R, Wanjer M, Vargas CR (2009) Amino acids levels and lipid peroxidation in maple syrup urine disease patients. Clin Biochem 42:462–466
Bickel H, Gerrard J, Hickmans EM (1953) Influence of phenylalanine intake on phenylketonuria. Lancet 265:812–813
Carmagnol F, Sinet PM, Jerome H (1983) Selenium-dependent and nonselenium-dependent glutathione peroxidases in human tissue extracts. Biochim Biophys Acta 759:49–57
Chace DH, Hillman SL, Van Hove JLK, Naylor EW (1997) Rapid diagnosis of MCAD deficiency: quantitative analysis of octanoylcarnitine and other acylcarnitines in newborn blood spots by tandem mass spectrometry. Clin Chem 43:2106–2113
Chaudière J (1994) Some chemical and biochemical constraints of oxidative stress in living cells. In: Riee-Evans CA, Burdon RH (eds) Free radical damage and its control. Elsevier, Amsterdam, pp 25–66
Colome C, Artuch R, Vilaseca MA, Sierra C, Brandi N, Lambruschini N, Cambra FJ, Campistol J (2003) Lipophilic antioxidants in patients with phenylketonuria. Am J Clin Nutr 77:185–188
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38
Dalle-Donne I, Scaloni A, Giustarini D, Cavarra E, Tell G, Lungarella G, Colombo R, Rossi R, Milzani A (2005) Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spectr Rev 24:55–99
Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A (2006) Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 10:389–406
Davies MJ, Fu S, Wang H, Dean RT (1999) Stable markers of oxidant damage to proteins and their application in study of human disease. Free Radic Biol Med 27:1151–1161
Deon M, Sitta A, Barschak AG, Coelho DM, Pigatto M, Schmitt GO, Jardim LB, Giugliani R, Wajner M, Vargas CR (2007) Induction of lipid peroxidation and decrease of antioxidant defenses in symptomatic and asymptomatic patients with X-linked adrenoleukodystrophy. Int J Dev Neurosci 25:441–447
Derin N, Izgut-Uysal VN, Agac A, Aliciguzel Y, Demir N (2004) l-carnitine protects gastric mucosa by decreasing ischemia reperfusion induced lipid peroxidation. J Physiol Pharmacol 55:595–606
Ercal N, Aykin-Burns N, Gurer-Orhan H, Mcdonald JD (2002) Oxidative stress in a phenylketonuria animal model. Free Radic Biol Med 32:906–911
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
German B (1999) Free radical and antioxidant protocols. Humana Press, Totowa
Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL (2008) The lipid Whisker model of the structure of oxidized cell membranes. J Biol Chem 283:2385–2396
Hagen MEK, Pederzolli CD, Sgaravatti AM, Bridi R, Wajner M, Wanmacher CMD, Wyse ATS, Dutra-Filho CS (2002) Experimental hyperphenylalaninemia provokes oxidative stress in rat brain. Biochim Biophys Acta 1586:344–352
Halliwell B, Gutteridge JMC (2007) Oxidative stress: adaptation, damage, repair and death. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press, Oxford, pp 246–350
Huttenlocher PR (2000) The neuropathology of phenylketonuria: human and animal studies. Eur J Pediatr 159:102–106
Lombeck I, Jochum F, Terwolbeck K (1996) Selenium status in infants and children with phenylketonuria and in maternal phenylketonuria. Eur J Pediatr 155:140–144
Lowry OH, Rosebrough NJ, Lewis-Farr A, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Markesbery WR, Lovell MA (2007) Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch Neurol 64:954–956
Martinez-Cruz F, Pozo D, Osuna C, Espinar A, Marchante C, Guerrero JM (2002) Oxidative stress induced by phenylketonuria in the rat: prevent by melatonin, vitamin E and vitamin C. J Neurosci Res 69:550–558
Mc Guire PJ, Parikh A, Diaz GA (2009) Profiling of oxidative stress in patients with inborn errors of metabolism. Mol Genet Metab 98:173–180
Paglia DE, Valentine WN (1967) Glutathione peroxidase. J Lab Clin Med 70:158
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Rajasekar P, Kaviarasan S, Anuradha CV (2005) l-carnitine administration prevents oxidative stress in high fructose-fed insulin resistant rats. Diab Croat 34:21–28
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Ribas GS, Manfredini V, de Mari JF, Wayhs CY, Vanzin CS, Biancini GB, Sitta A, Deon M, Wajner M, Vargas CR (2010) Reduction of lipid and protein damage in patients with disorders of propionate metabolism under treatment: a possible protective role of l-carnitine supplementation. Int J Dev Neurosci 28:127–132
Schulpis KH, Nounopoulos C, Scarpalezou A, Bouloukos A, Missiou-Tsagarakis S (1990) Serum carnitine level in phenylketonuric children under dietary control in Greece. Acta Paediatr Scand 79:930–934
Schulpis KH, Tsakiris S, Traeger-Synodinos J, Papassotiriou I (2005) Low total antioxidant status is implicated with high 8-hydroxy-2-deoxyguanosine serum concentrations in phenylketonuria. Clin Biochem 38:239–242
Scriver CR, Kaufman S (2001) Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1667–1724
Sierra C, Vilaseca MA, Moyano D, Brandi N, Campistol J, Lambruschini N, Cambra FJ, Deulofeu R, Mira A (1998) Antioxidant status in hyperphenylalaninemia. Clin Chim Acta 276:1–9
Sirtori LR, Dutra-Filho CS, Fitarelli D, Sitta A, Haeser A, Barschak AG, Wajner M, Coelho DM, Llesuy S, Belló-Klein A, Giugliani R, Deon M, Vargas CR (2005) Oxidative stress in patients with phenylketonuria. Biochim Biophys Acta 1740:68–73
Sitta A, Barschak AG, Deon M, Terroso T, Pires R, Giugliani R, Dutra-Filho CS, Wajner M, Vargas CR (2006) Investigation of oxidative stress parameters in treated phenylketonuric patients. Metab Brain Dis 21:287–296
Sitta A, Barschak AG, Deon M, Barden AT, Biancini GB, Vargas PR, de Souza CF, Netto C, Wajner M, Vargas CR (2009a) Effect of short- and long-term exposition to high phenylalanine blood levels on oxidative damage in phenylketonuric patients. Int J Dev Neurosci 27:243–247
Sitta A, Barschak AG, Deon M, De Mari JF, Barden AB, Vanzin C, Biancini GB, Schwartz IVD, Wajner M, Vargas CR (2009b) l-Carnitine blood levels and oxidative stress in treated phenylketonuric patients. Cell Mol Nurobiol 29:211–218
Smith DG, Cappai R, Barnham KJ (2007) The redox chemistry of the Alzheimer’s disease amyloid-b peptide. Biochim Biophys Acta 1768:1976–1990
Start K (1998) Treating phenylketonuria by a phenylalanine-free diet. Prof Care Mother Child 8:109–110
van Backel MME, Printzen G, Wermuth B, Wiesmann UN (2000) Antioxidant and thyroid hormone status in selenium-deficient phenylketonuric and hyperphenylalaninemic patients. Am J Clin Nutr 72:976–981
Van Spronsen FJ, Smit PG, Koch R (2001) Phenylketonuria: tyrosine beyond the phenylalanine-restricted diet. J Inherit Metab Dis 24:1–4
Wilke BC, Vidailhet M, Favier A, Guillemin C, Ducros V, Arnaud J, Richard MJ (1992) Selenium, glutathione peroxidase (GSH-Px) and lipid peroxidation products before and after selenium supplementation. Clin Chim Acta 207:137–142
Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, Kim K, Rhee SG (2003) Reversing the inactivation of peroxiredoxin caused by cysteine sulfinic acid formation. Science 300:53–656
Acknowledgments
This work was supported in part by grants from FAPERGS, CNPq, and FIPE/HCPA-Brazil.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sitta, A., Vanzin, C.S., Biancini, G.B. et al. Evidence that l-Carnitine and Selenium Supplementation Reduces Oxidative Stress in Phenylketonuric Patients. Cell Mol Neurobiol 31, 429–436 (2011). https://doi.org/10.1007/s10571-010-9636-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-010-9636-3