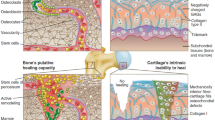
Abstract
Articular cartilage is the avascular and aneural tissue which is the primary connective tissue covering the surface of articulating bone. Traumatic damage or degenerative diseases can cause articular cartilage injuries that are common in the population. As a result, the demand for new therapeutic options is continually increasing for older people and traumatic young patients. Many attempts have been made to address these clinical needs to treat articular cartilage injuries, including osteoarthritis (OA); however, regenerating highly qualified cartilage tissue remains a significant obstacle. 3D bioprinting technology combined with tissue engineering principles has been developed to create biological tissue constructs that recapitulate the anatomical, structural, and functional properties of native tissues. In addition, this cutting-edge technology can precisely place multiple cell types in a 3D tissue architecture. Thus, 3D bioprinting has rapidly become the most innovative tool for manufacturing clinically applicable bioengineered tissue constructs. This has led to increased interest in 3D bioprinting in articular cartilage tissue engineering applications. Here, we reviewed current advances in bioprinting for articular cartilage tissue engineering.





Similar content being viewed by others
References
Xue, W., B. V. Krishna, A. Bandyopadhyay, and S. Bose. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 3:1007–1018, 2007.
Kim, J. H., J. J. Yoo, and S. J. Lee. Three-dimensional cell-based bioprinting for soft tissue regeneration. Tissue Eng. Regen. Med. 13:647–662, 2016.
Derby, B. Printing and prototyping of tissues and scaffolds. Science. 338:921–926, 2012.
Hockaday, L. A., K. H. Kang, N. W. Colangelo, P. Y. Cheung, B. Duan, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication.4:035005, 2012.
Ozbolat, I. T., and M. Hospodiuk. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 76:321–343, 2016.
Kang, H. W., S. J. Lee, I. K. Ko, C. Kengla, J. J. Yoo, and A. Atala. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34:312–319, 2016.
Moroni, L., J. A. Burdick, C. Highley, S. J. Lee, Y. Morimoto, et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 3:21–37, 2018.
Murphy, S. V., and A. Atala. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32:773–785, 2014.
Lee, M., B. M. Wu, and J. C. Dunn. Effect of scaffold architecture and pore size on smooth muscle cell growth. J. Biomed. Mater. Res. A. 87:1010–1016, 2008.
Tsang, V. L., and S. N. Bhatia. Three-dimensional tissue fabrication. Adv. Drug Deliv. Rev. 56:1635–1647, 2004.
Makris, E. A., A. H. Gomoll, K. N. Malizos, J. C. Hu, and K. A. Athanasiou. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 11:21–34, 2015.
Tatman, P. D., W. Gerull, S. Sweeney-Easter, J. I. Davis, A. O. Gee, and D. H. Kim. Multiscale biofabrication of articular cartilage: bioinspired and biomimetic approaches. Tissue Eng. Part B. 21:543–559, 2015.
Di Bella, C., A. Fosang, D. M. Donati, G. G. Wallace, and P. F. Choong. 3D bioprinting of cartilage for orthopedic surgeons: reading between the lines. Front Surg. 2:39, 2015.
Hjelle, K., E. Solheim, T. Strand, R. Muri, and M. Brittberg. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy. 18:730–734, 2002.
Curl, W. W., J. Krome, E. S. Gordon, J. Rushing, B. P. Smith, and G. G. Poehling. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 13:456–460, 1997.
Cibere, J., E. Sayre, A. Guermazi, S. Nicolaou, J. Kopec, et al. Natural history of cartilage damage and osteoarthritis progression on magnetic resonance imaging in a population-based cohort with knee pain. Osteoarthr. Cartil. 19:683–688, 2011.
Shelbourne, K. D., S. Jari, and T. Gray. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. JBJS. 85:8–16, 2003.
Lawrence, R. C., D. T. Felson, C. G. Helmick, L. M. Arnold, H. Choi, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States Part II. Arthritis Rheum. 58:26–35, 2008.
March, L. M., and C. J. Bachmeier. Economics of osteoarthritis: a global perspective. Baillieres Clin. Rheumatol. 11:817–834, 1997.
Hunziker, E. B., K. Lippuner, M. J. Keel, and N. Shintani. An educational review of cartilage repair: precepts & practice–myths & misconceptions–progress & prospects. Osteoarthr. Cartil. 23:334–350, 2015.
Van Blitterswijk, C., and J. De Boer. Tissue Engineering. Cambridge: Academic Press, 2014.
Niethammer, T. R., A. Loitzsch, A. Horng, A. Baur-Melnyk, M. Bendiks, et al. Graft hypertrophy after third-generation autologous chondrocyte implantation has no correlation with reduced cartilage quality: matched-pair analysis using T2-weighted mapping. Am. J. Sports Med. 46:2414–2421, 2018.
Martín, A. R., J. M. Patel, H. M. Zlotnick, J. L. Carey, and R. L. Mauck. Emerging therapies for cartilage regeneration in currently excluded “red knee” populations. NPJ Regen. Med. 4:12, 2019.
Jeznach, O., D. Kołbuk, and P. Sajkiewicz. Injectable hydrogels and nanocomposite hydrogels for cartilage regeneration. J. Biomed Mater. Res. A. 106:2762–2776, 2018.
Gadjanski, I. Recent advances on gradient hydrogels in biomimetic cartilage tissue engineering. F1000Res. 6:18, 2017.
Marjorie, D., S. Lilian, M. Marie, S. Matthieu, P. G. Emeline, S. Gilles, J. Christian, and Danièlenoël. 3D bioprinting of articular cartilage: recent advances and perspectives. Bioprinting. 28:15, 2022.
Pattappa, G., B. Johnstone, J. Zellner, D. Docheva, and P. Angele. The Importance of physioxia in mesenchymal stem cell chondrogenesis and the mechanisms controlling its response. Int. J. Mol. Sci. 20:18, 2019.
Ansari, S., S. Khorshidi, and A. Karkhaneh. Engineering of gradient osteochondral tissue: from nature to lab. Acta Biomater. 87:41–54, 2019.
Medvedeva, E. V., E. A. Grebenik, S. N. Gornostaeva, V. I. Telpuhov, A. V. Lychagin, et al. Repair of damaged articular cartilage: current approaches and future directions. Int J Mol Sci. 19:16, 2018.
Klein, T. J., J. Malda, R. L. Sah, and D. W. Hutmacher. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng. Part B. 15:143–157, 2009.
Armstrong, C., and V. C. Mow. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J. Bone Joint Surgery Am. 64:88–94, 1982.
Bhattarai, A., J. T. A. Mäkelä, B. Pouran, H. Kröger, H. Weinans, et al. Effects of human articular cartilage constituents on simultaneous diffusion of cationic and nonionic contrast agents. J. Orthop. Res. 39:771–779, 2021.
Alcaide-Ruggiero, L., V. Molina-Hernández, M. M. Granados, and J. M. Domínguez. Main and minor types of collagens in the articular cartilage: the role of collagens in repair tissue evaluation in chondral defects. Int. J. Mol. Sci. 22:26, 2021.
Kadler, K. E., C. Baldock, J. Bella, and R. P. Boot-Handford. Collagens at a glance. J. Cell Sci. 120:1955–1958, 2007.
Chung, C., and J. A. Burdick. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 60:243–262, 2008.
Posey, K. L., F. Coustry, and J. T. Hecht. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 71–72:161–173, 2018.
Knudson, C. B., and W. Knudson. Cartilage proteoglycans. Semin Cell. Dev. Biol. 12:69–78, 2001.
Fulcher, G. R., D. W. Hukins, and D. E. Shepherd. Viscoelastic properties of bovine articular cartilage attached to subchondral bone at high frequencies. BMC Musculoskelet. Disord. 10:1–7, 2009.
Jakob, M., O. Démarteau, R. Suetterlin, M. Heberer, and I. Martin. Chondrogenesis of expanded adult human articular chondrocytes is enhanced by specific prostaglandins. Rheumatology (Oxford). 43:852–857, 2004.
Walker, P. S., and J. V. Hajek. The load-bearing area in the knee joint. J. Biomech. 5:581–589, 1972.
Qian, Y., D. Hanhua, S. Jin, H. Jianhua, S. Bo, W. Qingsong, and S. Yusheng. A Review of 3D printing technology for medical applications. Engineering. 4:729–742, 2018.
Daniela, F. D. C., A. P. Midhun, G. Stefanie, M. Christoph, Y. L. Ying, S. Jan, B. Andreas, T. Benjamin, F. Horst, and B. Marcel. Synchronized dual bioprinting of bioinks and biomaterials inks as a translational strategy for cartilage tissue engineering. Print. Addit. Manuf. 6:63, 2019.
Aisenbrey, E. A., A. Tomaschke, E. Kleinjan, A. Muralidharan, C. Pascual-Garrido, et al. A stereolithography-based 3D printed hybrid scaffold for in situ cartilage defect repair. Macromol. Biosci. 18:29, 2018.
Liang, Q., Y. Ma, X. Yao, and W. Wei. Advanced 3D-printing bioinks for articular cartilage repair. Int. J. Bioprint. 8:511, 2022.
Sears, N. A., D. R. Seshadri, P. S. Dhavalikar, and E. Cosgriff-Hernandez. A review of three-dimensional printing in tissue engineering. Tissue Eng. Part B. 22:298–310, 2016.
Cui, X., T. Boland, D. D. D’Lima, and M. K. Lotz. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv. Formul. 6:149–155, 2012.
Zhang, X., X. Jiang, and C. Sun. Micro-stereolithography of polymeric and ceramic microstructures. Sens. Actuators A. 77:149–156, 1999.
Kim, S. H., Y. K. Yeon, J. M. Lee, J. R. Chao, Y. J. Lee, et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 9:1620, 2018.
Chan, V., P. Zorlutuna, J. H. Jeong, H. Kong, and R. Bashir. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab. Chip. 10:2062–2070, 2010.
Mauck, R., X. Yuan, and R. S. Tuan. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr. Cartil. 14:179–189, 2006.
da Silva, M. L., A. M. Fontes, D. T. Covas, and A. I. Caplan. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 20:419–427, 2009.
Caplan, A. I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 213:341–347, 2007.
Gibson, J. D., M. B. O’Sullivan, F. Alaee, D. N. Paglia, R. Yoshida, et al. Regeneration of articular cartilage by human ESC-derived mesenchymal progenitors treated sequentially with BMP-2 and Wnt5a. Stem Cells Transl. Med. 6:40–50, 2017.
Nguyen, D., D. A. Hägg, A. Forsman, J. Ekholm, P. Nimkingratana, et al. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep. 7:658, 2017.
Yi, Y., K. B. Choi, C.-L. Lim, J.-P. Hyun, H.-Y. Lee, et al. Irradiated human chondrocytes expressing bone morphogenetic protein 2 promote healing of osteoporotic bone fracture in rats. Tissue Eng. Part A. 15:2853–2863, 2009.
Hwang, N. S., S. Varghese, and J. Elisseeff. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS ONE.3:e2498, 2008.
Qu, C., K. A. Puttonen, H. Lindeberg, M. Ruponen, O. Hovatta, et al. Chondrogenic differentiation of human pluripotent stem cells in chondrocyte co-culture. Int. J. Biochem. Cell Biol. 45:1802–1812, 2013.
Chen, J., C. Wang, S. Lü, J. Wu, X. Guo, et al. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 319:429–438, 2005.
Boeuf, S., and W. Richter. Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Res. Ther. 1:31, 2010.
Pleumeekers, M. M., L. Nimeskern, J. L. M. Koevoet, M. Karperien, K. S. Stok, and G. J. V. M. van Osch. Trophic effects of adipose-tissue-derived and bone-marrow-derived mesenchymal stem cells enhance cartilage generation by chondrocytes in co-culture. PLoS ONE.13:e0190744, 2018.
Mahmoudifar, N., and P. M. Doran. Chondrogenic differentiation of human adipose-derived stem cells in polyglycolic acid mesh scaffolds under dynamic culture conditions. Biomaterials. 31:3858–3867, 2010.
Chang, C. H., C. C. Chen, C. H. Liao, F. H. Lin, Y. M. Hsu, and H. W. Fang. Human acellular cartilage matrix powders as a biological scaffold for cartilage tissue engineering with synovium-derived mesenchymal stem cells. J. Biomed. Mater. Res. Part A. 102:2248–2257, 2014.
Baksh, D., R. Yao, and R. S. Tuan. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 25:1384–1392, 2007.
Nejadnik, H., J. H. Hui, E. P. Feng Choong, B. C. Tai, and E. H. Lee. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am. J. Sports Med. 38:1110–1116, 2010.
De Bari, C., F. Dell’Accio, P. Tylzanowski, and F. P. Luyten. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheumatism. 44:1928–1942, 2001.
De Bari, C., and A. J. Roelofs. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 40:74–80, 2018.
Park, Y., C. Ha, J. Kim, W. Han, J. Rhim, et al. Single-stage cell-based cartilage repair in a rabbit model: cell tracking and in vivo chondrogenesis of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel composite. Osteoarthr. Cartil. 25:570–580, 2017.
Somoza, R. A., J. F. Welter, D. Correa, and A. I. Caplan. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng. Part B. 20:596–608, 2014.
Chen, W., C. Li, M. Peng, B. Xie, L. Zhang, and X. Tang. Autologous nasal chondrocytes delivered by injectable hydrogel for in vivo articular cartilage regeneration. Cell Tissue Bank. 19:35–46, 2018.
Vinatier, C., O. Gauthier, M. Masson, O. Malard, A. Moreau, et al. Nasal chondrocytes and fibrin sealant for cartilage tissue engineering. J. Biomed. Mater. Re.s A. 89:176–185, 2009.
Mumme, M., A. Barbero, S. Miot, A. Wixmerten, S. Feliciano, et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 388:1985–1994, 2016.
Raul Sanchez-Sanchez, J.M.R.-R., Antoni Macias-Garcia, Laura Mendoza-Cerezo, and Antonio Diaz-Parralejo. Relationship between shear-thinning rheological properties of bioinks and bioprinting parameters. Int. J. Bioprint. 9:422, 2023.
Habib, M. A., and B. Khoda. Rheological analysis of bio-ink for 3D bio-printing processes. J. Manuf. Process. 76:708–718, 2022.
Lee, V., G. Singh, J. P. Trasatti, C. Bjornsson, X. Xu, et al. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C. 20:473–484, 2014.
Song, S. J., J. Choi, Y. D. Park, J. J. Lee, S. Y. Hong, and K. Sun. A three-dimensional bioprinting system for use with a hydrogel-based biomaterial and printing parameter characterization. Artif. Organs. 34:1044–1048, 2010.
Almeida, C. R., T. Serra, M. I. Oliveira, J. A. Planell, M. A. Barbosa, and M. Navarro. Impact of 3-D printed PLA-and chitosan-based scaffolds on human monocyte/macrophage responses: unraveling the effect of 3-D structures on inflammation. Acta Biomater. 10:613–622, 2014.
Khalil, S., and W. Sun. Bioprinting endothelial cells with alginate for 3D tissue constructs. J. Biomech. Eng. 131:15, 2009.
Daly, A. C., S. E. Critchley, E. M. Rencsok, and D. J. Kelly. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication.8:045002, 2016.
Kundu, J., J. H. Shim, J. Jang, S. W. Kim, and D. W. Cho. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 9:1286–1297, 2015.
Visser, J., F. P. Melchels, J. E. Jeon, E. M. Van Bussel, L. S. Kimpton, et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 6:1–10, 2015.
Xu, T., K. W. Binder, M. Z. Albanna, D. Dice, W. Zhao, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication.5:015001, 2013.
Müller, M., E. Öztürk, Ø. Arlov, P. Gatenholm, and M. Zenobi-Wong. Alginate sulfate-nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 45:210–223, 2017.
Skardal, A., M. Devarasetty, H. W. Kang, I. Mead, C. Bishop, et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 25:24–34, 2015.
Roseti, L., C. Cavallo, G. Desando, V. Parisi, M. Petretta, et al. Three-dimensional bioprinting of cartilage by the use of stem cells: a strategy to improve regeneration. Materials (Basel). 11:25, 2018.
Pati, F., J. Jang, D. H. Ha, S. Won Kim, J. W. Rhie, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5:3935, 2014.
Yang, S. S., W. H. Choi, B. R. Song, H. Jin, S. J. Lee, et al. Fabrication of an osteochondral graft with using a solid freeform fabrication system. Tissue Eng. Regenerat. Med. 12:239–248, 2015.
Ali, M., A. K. Pr, J. J. Yoo, F. Zahran, A. Atala, and S. J. Lee. A photo-crosslinkable kidney ECM-derived bioink accelerates renal tissue formation. Adv. Healthc. Mater.8:e1800992, 2019.
Visscher, D. O., H. Lee, P. P. M. van Zuijlen, M. N. Helder, A. Atala, et al. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater. 121:193–203, 2021.
Lee, H., W. Kim, J. Lee, K. S. Park, J. J. Yoo, et al. Self-aligned myofibers in 3D bioprinted extracellular matrix-based construct accelerate skeletal muscle function restoration. Appl. Phys. Rev.8:021405, 2021.
Song, B. R., S. S. Yang, H. Jin, S. H. Lee, D. Y. Park, et al. Three dimensional plotted extracellular matrix scaffolds using a rapid prototyping for tissue engineering application. Tissue Eng. Regenerat. Med. 12:172–180, 2015.
Jung, C. S., B. K. Kim, J. Lee, B.-H. Min, and S.-H. Park. Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape. Tissue Eng. Regenerat. Med. 15:155–162, 2018.
Zhang, X., Y. Liu, C. Luo, C. Zhai, Z. Li, et al. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater. Sci. Eng. C.118:111388, 2021.
Mwale, F., D. Stachura, P. Roughley, and J. Antoniou. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J. Orthop. Res. 24:1791–1798, 2006.
Yoon, B. S., and K. M. Lyons. Multiple functions of BMPs in chondrogenesis. J. Cell Biochem. 93:93–103, 2004.
Goldring, M. B., K. Tsuchimochi, and K. Ijiri. The control of chondrogenesis. J. Cell Biochem. 97:33–44, 2006.
Bouffi, C., O. Thomas, C. Bony, A. Giteau, M. C. Venier-Julienne, et al. The role of pharmacologically active microcarriers releasing TGF-beta3 in cartilage formation in vivo by mesenchymal stem cells. Biomaterials. 31:6485–6493, 2010.
Lee, C. H., S. A. Rodeo, L. A. Fortier, C. Lu, C. Erisken, and J. J. Mao. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci. Transl. Med. 6:266ra171, 2014.
van der Kraan, P. M., J. de Lange, E. L. Vitters, H. M. van Beuningen, G. J. van Osch, et al. Analysis of changes in proteoglycan content in murine articular cartilage using image analysis. Osteoarthr. Cartil. 2:207–214, 1994.
Li KW, Klein TJ, Chawla K, Nugent GE, Bae WC, Sah RL. 2004. In vitro physical stimulation of tissue-engineered and native cartilage. In Cartilage and osteoarthritis:325–51: Springer. Number of 325–51 pp.
Lima, E. G., R. L. Mauck, S. H. Han, S. Park, K. W. Ng, et al. Functional tissue engineering of chondral and osteochondral constructs. Biorheology. 41:577–590, 2004.
Grad, S., D. Eglin, M. Alini, and M. J. Stoddart. Physical stimulation of chondrogenic cells in vitro: a review. Clin. Orthop. Relat. Res. 469:2764–2772, 2011.
Fu, L., P. Li, H. Li, C. Gao, Z. Yang, et al. The application of bioreactors for cartilage tissue engineering: advances, limitations, and future perspectives. Stem Cells Int. 2021:6621806, 2021.
Kengla, C., E. Renteria, C. Wivell, A. Atala, J. J. Yoo, and S. J. Lee. Clinical relevant bioprinting workflow and imaging process for tissue construct design and validation. 3D Printing Manufac. 4:239–247, 2017.
Cui, X., K. Breitenkamp, M. Finn, M. Lotz, and D. D. D’Lima. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A. 18:1304–1312, 2012.
Ma, K., T. Zhao, L. Yang, P. Wang, J. Jin, et al. Application of robotic-assisted in situ 3D printing in cartilage regeneration with HAMA hydrogel: an in vivo study. J. Adv. Res. 23:123–132, 2020.
Li, L., F. Yu, J. Shi, S. Shen, H. Teng, et al. In situ repair of bone and cartilage defects using 3D scanning and 3D printing. Sci. Rep. 7:9416, 2017.
Samandari, M., A. Mostafavi, J. Quint, A. Memic, and A. Tamayol. In situ bioprinting: intraoperative implementation of regenerative medicine. Trends Biotechnol. 40:1229–1247, 2022.
Behan, K., A. Dufour, O. Garcia, and D. Kelly. Methacrylated cartilage ecm-based hydrogels as injectables and bioinks for cartilage tissue engineering. Biomolecules. 12:15, 2022.
Zhu, S., P. Chen, Y. Chen, M. Li, C. Chen, and H. Lu. 3D-Printed Extracellular matrix/polyethylene glycol diacrylate hydrogel incorporating the anti-inflammatory phytomolecule honokiol for regeneration of osteochondral defects. Am. J. Sports Med. 48:2808–2818, 2020.
Acknowledgements
This review was supported by the National Institutes of Health (1P41EB023833-01), the National Science Foundation (2100739), and the National Research Foundation of Korea (NRF-2022R1A2C1091873).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that any no conflict of interest
Additional information
Associate Editor Emmanuel Opara oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, D.Y., Kim, SH., Park, SH. et al. 3D Bioprinting Strategies for Articular Cartilage Tissue Engineering. Ann Biomed Eng (2023). https://doi.org/10.1007/s10439-023-03236-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-023-03236-8