Abstract
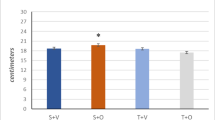
We evaluated whether strength training (ST) performed prior to skeletal muscle cryolesion would act as a preconditioning, improving skeletal muscle regeneration and responsiveness to low-level laser therapy (LLLT). Wistar rats were randomly assigned into non-exercised (NE), NE plus muscle lesion (NE + LE), NE + LE plus LLLT (NE + LE + LLLT), strength training (ST), ST + LE, and ST + LE + LLLT. The animals performed 10 weeks of ST (climbing ladder; 3× week; 80% overload). Forty-eight hours after the last ST session, tibialis anterior (TA) cryolesion was induced and LLLT (InGaAlP, 660 nm, 0.035 W, 4.9 J/cm2/point, 3 points, spot light 0.028 cm2, 14 J/cm2) initiated and conducted daily for 14 consecutive days. The difference between intergroups was assessed using Student’s t test and intragroups by two-way analysis of variance. Cryolesion induced massive muscle degeneration associated with inflammatory infiltrate. Prior ST improved skeletal regeneration 14-days after cryolesion and potentiated the regenerative response to LLLT. Cryolesion induced increased TNF-α levels in both NE + LE and ST + LE groups. Both isolated ST and LLLT reduced TNF-α to control group levels; however, prior ST potentiated LLLT response. Both isolated ST and LLLT increased IL-10 levels with no additional effect. In contrast, increased TA IL-6 levels were restricted to ST and ST + LE + LLLT groups. TA myogenin mRNA levels were not changed by neither prior ST or ST + LLLT. Both prior ST and LLLT therapies increased MyoD mRNA levels and, interestingly, combined therapies potentiated this response. Myf5 mRNA levels were increased only in ST groups. Taken together, our data provides evidences for prior ST potentiating LLLT efficacy in promoting skeletal muscle regeneration.





Similar content being viewed by others
References
Ribeiro BG, Alves AN, Santos LA, Fernandes KP, Cantero TM et al (2015) The effect of low-level laser therapy (LLLT) applied prior to muscle injury. Lasers Surg Med 47:571–78
de Melo CA, Alves AN, Terena SM, Fernandes KP, Nunes FD et al (2015) Light-emitting diode therapy increases collagen deposition during the repair process of skeletal muscle. Lasers Med Sci 31:531–8
Musarò A (2014) The Basis of Muscle Regeneration. Adv Biol 1–16
Fukuda TY, Tanji MM, Jesus JF, Sato NM, Duarte AJ et al (2010) Single session to infrared low level diode laser on TNF-α and IL-6 cytokines release by mononuclear spleen cells in mice: A pilot study. Lasers Surg Med 42:584–588
Pires D, Xavier M, Araújo T, Silva JA Jr, Aimbire F et al (2011) Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 26:85–94
Dinarello CA (2000) Proinflammatory cytokines. Chest 118:503–508
Ostrowski K, Schjerling P, Pedersen BK (2000) Physical activity and plasma interleukin-6 in humans—effect of intensity of exercise. Eur J Appl Physiol 83:512–5
Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK (1999) Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515:287–291
Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ (2000) MyoD (−/−) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev Biol 224:122–137
Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW (1992) Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res 267:99–104
Füchtbauer EM, Westphal H (1992) MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Dev Dyn 193:34–9
Tidball JG, Wehling-Henricks M (2007) Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol 578:327–336
Tidball JG, Villalta SA (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298:R1173–87
Leal-Junior EC, de Almeida P, Tomazoni SS, de Carvalho PT, Lopes-Martins RÁ et al (2014) Superpulsed low-level laser therapy protects skeletal muscle of mdx mice against damage, inflammation and morphological changes delaying dystrophy progression. PLoS One 9, e89453
Alves AN, Fernandes KP, Deana AM, Bussadori SK, Mesquita-Ferrari RA (2014) Effects of low-level laser therapy on skeletal muscle repair: a systematic review. Am J Phys Med Rehabil 93:1073–85
Baptista J, Martins MD, Pavesi VC, Bussadori SK, Fernandes KP et al (2011) Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomed Laser Surg 29:11–7
Assis L, Moretti AI, Abrahão TB, de Souza HP, Hamblin MR et al (2013) Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci 28:947–955
Larkin-Kaiser KA, Christou E, Tillman M, George S, Borsa PA et al (2015) Near-infrared light therapy to attenuate strength loss after strenuous resistance exercise. J Athl Train 50:45–50
Assis L, Yamashita F, Magri AM, Fernandes KR, Yamauchi L et al (2015) Effect of low-level laser therapy (808 nm) on skeletal muscle after endurance exercise training in rats. Braz J Phys Ther 19:457–465
Patrocinio T, Sardim AC, Assis L, Fernandes KR, Rodrigues N et al (2013) Effect of low-level Laser therapy (808 nm) in skeletal muscle after resistance exercise training in rats. Photomed Laser Surg 31:492–498
Toma RL, Tucci HT, Antunes HK, Pedroni CR, de Oliveira AS et al (2013) Effect of 808 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in elderly women. Lasers Med Sci 28:1375–82
Assis L, Almeida T, Milares LP, dos Passos N, Araújo B et al. (2015) Musculoskeletal Atrophy in an Experimental Model of Knee Osteoarthritis: The Effects of Exercise Training and Low-Level Laser Therapy. Am J Phys Med Rehabil 609–616
Hornberger TA Jr, Farrar RP (2004) Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol 29:16–31
Alves JP, Nunes RB, Stefani GP, Dal Lago P (2014) Resistance training improves hemodynamic function, collagen deposition and inflammatory profiles: experimental model of heart failure. PLoS One 9, e110317
Wiggs MP (2015) Can endurance exercise preconditioning prevention disuse muscle atrophy? Front Physiol 6:63
Stringhetta-Garcia CT, Singulani MP, Santos LF, Louzada MJ, Nakamune AC et al (2016) The effects of strength training and raloxifene on bone health in aging ovariectomized rats. Bone 85:45–54
Miyabara EH, Martin JL, Griffin TM, Moriscot AS, Mestril R (2006) Overexpression of inducible 70-kDa heat shock protein in mouse attenuates skeletal muscle damage induced by cryolesioning. Am J Physiol Cell Physiol 290:C1128–38
Jung S, Ahn N, Kim S, Byun J, Joo Y et al (2015) The effect of ladder-climbing exercise on atrophy/hypertrophy- related myokine expression in middle-aged male Wistar rats. J Physiol Sci 65:515–521
Rodrigues NC, Brunelli R, de Araújo HS, Parizotto NA, Renno AC (2013) Low-level laser therapy (LLLT) (660nm) alters gene expression during muscle healing in rats. J Photochem Photobiol B 120:29–35
Fernandes KP, Alves AN, Nunes FD, Souza NH, Silva JA Jr et al (2012) Effect of photobiomodulation on expression of IL-1β in skeletal muscle following acute injury. Lasers Med Sci 28:1043–1046
Rennó AC, Toma RL, Feitosa SM, Fernandes K, Bossini PS et al (2011) Comparative effects of low-intensity pulsed ultrasound and low-level laser therapy on injured skeletal muscle. Photomed Laser Surg 29:5–10
Liu XG, Zhou YJ, Liu TC, Yuan JQ (2009) Effects of low-level laser irradiation on rat skeletal muscle injury after eccentric exercise. Photomed Laser Surg 27:863–869
Rodrigues NC, Brunelli R, de Araújo HS, Parizotto NA, Renno AC (2013) Low-level laser therapy (LLLT) (660 nm) alters gene expression during muscle healing in rats. J Photochem Photobiol B Biol 120:29–35
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25:402–408
Alves AN, Ribeiro BG, Fernandes KP, Souza NH, Rocha LA et al (2016) Comparative effects of low-level laser therapy pre- and post-injury on mRNA expression of MyoD, myogenin, and IL-6 during the skeletal muscle repair. Lasers Med Sci 31:679–85
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol (1985) 91:534–51
Ferraresi C, de Brito Oliveira T, de Zafalon Oliveira L, de Menezes Reiff RB, Baldissera V et al (2011) Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers Med Sci 26:349–58
Leal-Junior EC, Vanin AA, Miranda EF, de Carvalho PT, Dal Corso S et al (2015) Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med Sci 30:925–939
Assis L, Milares LP, Almeida T, Tim C, Magri A et al (2016) Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr Cartil 24:169–177
Assis L, Moretti AI, Abrahão TB, Cury V, Souza HP et al (2012) Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Lasers Surg Med 44:726–735
Kurosaka M, Machida S (2013) Interleukin-6-induced satellite cell proliferation is regulated by induction of the JAK2/STAT3 signalling pathway through cyclin D1 targeting. Cell Prolif 46:365–373
Hoene M, Runge H, Häring HU, Schleicher ED, Weigert C (2013) Interleukin-6 promotes myogenic differentiation of mouse skeletal muscle cells: role of the STAT3 pathway. Am J Physiol Cell Physiol 304:C128–36
Pedersen BK (2007) IL-6 signalling in exercise and disease. Biochem Soc Trans 35:1295–1297
Acknowledgements
The authors would like to thank to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) by the financial support and scholarship (FAPESP Grant n° 2013/18907-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Faculty of Dentistry (Univ. Estadual Paulista—UNESP, Araçatuba, Sao Paulo, Brazil) (ethical committee approval—process no. 2013/01543) and complied with guidelines of the Brazilian National Council for the Control of Animal Experimentation. The authors declare that this manuscript was prepared in accordance to the “Ethical Responsibilities of Authors” and it is an unpublished material which it is not being evaluated for publication in other journals. The manuscript has been read by all authors and the contributions performed by each one were approved. This study received financial support from the Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (FAPESP Grant n° 2013/18907-2).
Conflict of interest
The authors declare no conflict of interest in this study.
Rights and permissions
About this article
Cite this article
Morais, S.R.L., Goya, A.G., Urias, Ú. et al. Strength training prior to muscle injury potentiates low-level laser therapy (LLLT)-induced muscle regeneration. Lasers Med Sci 32, 317–325 (2017). https://doi.org/10.1007/s10103-016-2116-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2116-3