Abstract
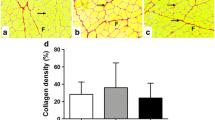
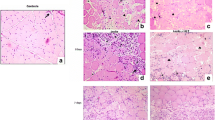
Muscle regeneration is a complex phenomenon, involving replacement of damaged fibers by new muscle fibers. During this process, there is a tendency to form scar tissue or fibrosis by deposition of collagen that could be detrimental to muscle function. New therapies that could regulate fibrosis and favor muscle regeneration would be important for physical therapy. Low-level laser therapy (LLLT) has been studied for clinical treatment of skeletal muscle injuries and disorders, even though the molecular and cellular mechanisms have not yet been clarified. The aim of this study was to evaluate the effects of LLLT on molecular markers involved in muscle fibrosis and regeneration after cryolesion of the tibialis anterior (TA) muscle in rats. Sixty Wistar rats were randomly divided into three groups: control, injured TA muscle without LLLT, injured TA muscle treated with LLLT. The injured region was irradiated daily for four consecutive days, starting immediately after the lesion using an AlGaAs laser (808 nm, 30 mW, 180 J/cm2; 3.8 W/cm2, 1.4 J). The animals were sacrificed on the fourth day after injury. LLLT significantly reduced the lesion percentage area in the injured muscle (p < 0.05), increased mRNA levels of the transcription factors MyoD and myogenin (p < 0.01) and the pro-angiogenic vascular endothelial growth factor (p < 0.01). Moreover, LLLT decreased the expression of the profibrotic transforming growth factor TGF-β mRNA (p < 0.01) and reduced type I collagen deposition (p < 0.01). These results suggest that LLLT could be an effective therapeutic approach for promoting skeletal muscle regeneration while preventing tissue fibrosis after muscle injury.





Similar content being viewed by others
References
Huard J, Li Y, Fu FH (2002) Muscle injuries and repair: current trends in research. J Bone Joint Surg Am 84-A(5):822–832
Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M (2005) Muscle injuries: biology and treatment. Am J Sports Med 33(5):745–764. doi:10.1177/0363546505274714
Filippin LI, Cuevas MJ, Lima E, Marroni NP, Gonzalez-Gallego J, Xavier RM (2011) Nitric oxide regulates the repair of injured skeletal muscle. Nitric Oxide 24(1):43–49. doi:10.1016/j.niox.2010.11.003
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84(1):209–238. doi:10.1152/physrev.00019.2003
Ehrhardt J, Morgan J (2005) Regenerative capacity of skeletal muscle. Curr Opin Neurol 18(5):548–553
Le Grand F, Rudnicki MA (2007) Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol 19(6):628–633. doi:10.1016/j.ceb.2007.09.012
Sakuma K, Watanabe K, Sano M, Uramoto I, Sakamoto K, Totsuka T (1999) The adaptive response of MyoD family proteins in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta 1428(2–3):284–292
Shi X, Garry DJ (2006) Muscle stem cells in development, regeneration, and disease. Genes Dev 20(13):1692–1708. doi:10.1101/gad.1419406
Rantanen J, Hurme T, Lukka R, Heino J, Kalimo H (1995) Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest 72(3):341–347
Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, Simeonova PP (2002) Physiological role of tumor necrosis factor alpha in traumatic muscle injury. FASEB J 16(12):1630–1632. doi:10.1096/fj.02-0187fje
Deveci D, Marshall JM, Egginton S (2002) Chronic hypoxia induces prolonged angiogenesis in skeletal muscles of rat. Exp Physiol 87(3):287–291
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7(5):359–371. doi:10.1038/nrm1911
Botusan IR, Sunkari VG, Savu O, Catrina AI, Grunler J, Lindberg S, Pereira T, Yla-Herttuala S, Poellinger L, Brismar K, Catrina SB (2008) Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA 105(49):19426–19431. doi:10.1073/pnas.0805230105
Wagatsuma A (2007) Endogenous expression of angiogenesis-related factors in response to muscle injury. Mol Cell Biochem 298(1–2):151–159. doi:10.1007/s11010-006-9361-x
Kollias HD, McDermott JC (2008) Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol 104(3):579–587. doi:10.1152/japplphysiol.01091.2007
Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J (2004) Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164(3):1007–1019
Hamblin MR (2010) Introduction to experimental and clinical studies using low-level laser (light) therapy (LLLT). Lasers Surg Med 42(6):447–449. doi:10.1002/lsm.20959
Demidova-Rice TN, Salomatina EV, Yaroslavsky AN, Herman IM, Hamblin MR (2007) Low-level light stimulates excisional wound healing in mice. Lasers Surg Med 39(9):706–715. doi:10.1002/lsm.20549
Ben-Dov N, Shefer G, Irintchev A, Wernig A, Oron U, Halevy O (1999) Low-energy laser irradiation affects satellite cell proliferation and differentiation in vitro. Biochim Biophys Acta 1448(3):372–380
Shefer G, Barash I, Oron U, Halevy O (2003) Low-energy laser irradiation enhances de novo protein synthesis via its effects on translation-regulatory proteins in skeletal muscle myoblasts. Biochim Biophys Acta 1593(2–3):131–139
Renno AC, Toma RL, Feitosa SM, Fernandes K, Bossini PS, de Oliveira P, Parizotto N, Ribeiro DA (2011) Comparative effects of low-intensity pulsed ultrasound and low-level laser therapy on injured skeletal muscle. Photomed Laser Surg 29(1):5–10. doi:10.1089/pho.2009.2715
Mesquita-Ferrari RA, Martins MD, Silva JA Jr, da Silva TD, Piovesan RF, Pavesi VC, Bussadori SK, Fernandes KP (2011) Effects of low-level laser therapy on expression of TNF-alpha and TGF-beta in skeletal muscle during the repair process. Lasers Med Sci 26(3):335–340. doi:10.1007/s10103-010-0850-5
Iyomasa DM, Garavelo I, Iyomasa MM, Watanabe IS, Issa JP (2009) Ultrastructural analysis of the low level laser therapy effects on the lesioned anterior tibial muscle in the gerbil. Micron 40(4):413–418. doi:10.1016/j.micron.2009.02.002
Tuby H, Maltz L, Oron U (2006) Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med 38(7):682–688. doi:10.1002/lsm.20377
Amaral AC, Parizotto NA, Salvini TF (2001) Dose-dependency of low-energy HeNe laser effect in regeneration of skeletal muscle in mice. Lasers Med Sci 16(1):44–51
Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R (2009) Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B 95(2):89–92. doi:10.1016/j.jphotobiol.2009.01.004
Nakano J, Kataoka H, Sakamoto J, Origuchi T, Okita M, Yoshimura T (2009) Low-level laser irradiation promotes the recovery of atrophied gastrocnemius skeletal muscle in rats. Exp Physiol 94(9):1005–1015. doi:10.1113/expphysiol.2009.047738
Cressoni MD, Dib Giusti HH, Casarotto RA, Anaruma CA (2008) The effects of a 785-nm AlGaInP laser on the regeneration of rat anterior tibialis muscle after surgically-induced injury. Photomed Laser Surg. doi:10.1089/pho.2007.2150
Miyabara EH, Martin JL, Griffin TM, Moriscot AS, Mestril R (2006) Overexpression of inducible 70-kDa heat shock protein in mouse attenuates skeletal muscle damage induced by cryolesioning. Am J Physiol Cell Physiol 290(4):C1128–C1138. doi:10.1152/ajpcell.00399.2005
Servetto N, Cremonezzi D, Simes JC, Moya M, Soriano F, Palma JA, Campana VR (2010) Evaluation of inflammatory biomarkers associated with oxidative stress and histological assessment of low-level laser therapy in experimental myopathy. Lasers Surg Med 42(6):577–583. doi:10.1002/lsm.20910
Holterman CE, Rudnicki MA (2005) Molecular regulation of satellite cell function. Semin Cell Dev Biol 16(4–5):575–584. doi:10.1016/j.semcdb.2005.07.004
Hurme T, Kalimo H (1992) Activation of myogenic precursor cells after muscle injury. Med Sci Sports Exerc 24(2):197–205
Shefer G, Oron U, Irintchev A, Wernig A, Halevy O (2001) Skeletal muscle cell activation by low-energy laser irradiation: a role for the MAPK/ERK pathway. J Cell Physiol 187(1):73–80. doi:10.1002/1097-4652(2001)9999:9999<::AID-JCP1053>3.0.CO;2-9
Shefer G, Partridge TA, Heslop L, Gross JG, Oron U, Halevy O (2002) Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci 115(Pt 7):1461–1469
Gomes AR, Soares AG, Peviani S, Nascimento RB, Moriscot AS, Salvini TF (2006) The effect of 30 min of passive stretch of the rat soleus muscle on the myogenic differentiation, myostatin, and atrogin-1 gene expressions. Arch Phys Med Rehabil 87(2):241–246. doi:10.1016/j.apmr.2005.08.126
Oron U (2006) Photoengineering of tissue repair in skeletal and cardiac muscles. Photomed Laser Surg 24(2):111–120. doi:10.1089/pho.2006.24.111
Bibikova A, Belkin V, Oron U (1994) Enhancement of angiogenesis in regenerating gastrocnemius muscle of the toad (Bufo viridis) by low-energy laser irradiation. Anat Embryol (Berl) 190(6):597–602
Kipshidze N, Nikolaychik V, Keelan MH, Shankar LR, Khanna A, Kornowski R, Leon M, Moses J (2001) Low-power helium: neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro. Lasers Surg Med 28(4):355–364. doi:10.1002/lsm.1062
Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J (2001) The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med 29(4):394–402
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, Gonzalez-Gallego J (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37(4):293–300. doi:10.1002/lsm.20225
Rizzi CF, Mauriz JL, Freitas Correa DS, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, Gonzalez-Gallego J (2006) Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med 38(7):704–713. doi:10.1002/lsm.20371
Acknowledgments
We acknowledge CAPES, CNPQ and FAPESP for financial support. MR Hamblin was supported by NIH (grant R01AI050875). Emergency Medicine Division (LIM 51), Faculdade de Medicina da Universidade de São Paulo to provide technical support in biochemical and molecular biology analyses and NUPEN (Núcleo de Pesquisa e Ensino em Fototerapia nas Ciências da Saúde) for supporting and calibrating the laser equipment.
Disclosure of interests
The authors indicate no potential conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Assis, L., Moretti, A.I.S., Abrahão, T.B. et al. Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci 28, 947–955 (2013). https://doi.org/10.1007/s10103-012-1183-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1183-3