Abstract
Photoperiod (day-length) response, vernalization (response to extended periods of cold) and earliness per se (Eps) genes regulate flowering time in wheat. The vernalization and photoperiod response genes are relatively well studied. However, the role of Eps genes is yet to be fully understood but the current assumption is that Eps genes regulate flowering independent of vernalization and photoperiod. While some Eps genes have been cloned in both Hordeum vulgare and Triticum monococcum, none has been cloned in Triticum aestivum to date. The use of near isogenic lines (NILs) in both T. monococcum and Triticum aestivum has enabled Eps effects to be studied in more detail and candidate genes have been proposed for Eps effects in both species. Eps loci are reported to be involved in fine tuning flowering time and are also responsible for controlling spikelet number and size hence could be manipulated to increase wheat yield. This mini review summarises our current understanding of Eps and how manipulation of Eps genes can be used in predictive wheat breeding.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The world population demands more food, greater diversity of food, a balanced and healthy diet, produced on no more, and preferably less land, while conserving soil, water, and genetic resources. The major problem is that even though wheat yields are increasing (Lopes et al. 2012), the percentage increase is below the projected percentage demand with about 0.6 % deficit projected annually until 2050 (Dixon et al. 2009; Rosegrant and Agcaoili 2010). The challenge wheat breeders face is to bridge the gap between wheat demand and wheat production. It is therefore vital to direct wheat breeding efforts to the production of higher yielding varieties in order to ensure current and future food security (Reyolds et al. 2012). The part of the wheat plant that is important for direct consumption by humans is the grain and its production is dependent on flowering time. Manipulating flowering time is one avenue that can be exploited to increase wheat grain yield (Herndl et al. 2008; Greenup et al. 2009). However, in order to successfully increase grain yield, it is vital to thoroughly understand the genetic and physiological factors affecting wheat growth and development particularly flowering time genes (Gill et al. 2004).
There are three major classes of flowering time genes which are photoperiod (Ppd) response genes, vernalization (Vrn) response genes and earliness per se genes (Eps). Major photoperiod response genes enable wheat plants to perceive changes in day length with accelerated flowering occurring in long days while short days cause delayed flowering unless there are mutations in the PHOTOPERIOD 1 (Ppd-1) genes (Beales et al. 2007; Wilhelm et al. 2009; Dı’az et al. 2012). Three main genes VERNALIZATION 1, 2 and 3 (VRN1, VRN2 and VRN3) control the vernalization response in wheat (Yan et al. 2003; Trevaskis et al. 2007; Distelfeld et al. 2009a, b; Shimada et al. 2009; Distelfeld and Dubcovsky 2010; Dı’az et al. 2012). Wild type wheat require extended exposure to cold (known as winter wheat) before the transition from vegetative to reproductive growth while mutants do not require this exposure and are regarded as spring wheat (Fu et al. 2005).
The third class of genes controlling flowering time is earliness per se, also referred to as ear emergence per se, earliness in narrow sense, intrinsic earliness, and at times is called basic development rate (Laurie et al. 2004; Cockram et al. 2007; Shitsukawa et al. 2007; Lewis et al. 2008). A number of similar definitions have been proposed for Eps. Eps can be defined as the minimum number of days to reproductive growth, after vernalization and photoperiod requirements are satisfied (van Beem et al. 2005). Similarly, Appendino et al. (2003) defined Eps as the time to heading after both vernalization and photoperiod requirements are satisfied. Shitsukawa et al. (2007) defined narrow sense earliness or earliness per se as the earliness of fully vernalized plants grown under long days. Lewis et al. (2008) described Eps as all other genes controlling flowering time but not involved in either vernalization or photoperiod requirements. The Eps definitions suggest that these genes regulate flowering independent of both vernalization or photoperiod environmental cues (Bullrich et al. 2002).
The course and fine adjustment knobs of a light microscope can be used to visualise the role of the Eps genes in flowering time (Fig. 39.1). The Ppd and Vrn genes would be equivalent to the course adjustment knob and are responsible for adaptation to mega environments for example spring and winter wheat as well as short day and long day environments (Worland et al. 1994, 1998). The Eps genes are equivalent to the fine adjustment knob (Fig. 39.1) and are responsible for fine-tuning of wheat flowering time (Valarik et al. 2006) within mega-environments (Griffiths et al. 2009) and are responsible for wide adaptation of wheat to different environments (Lewis et al. 2008). Laurie et al. (2004) suggested that Eps factors may be largely responsible for the variation in flowering time in crosses within winter or spring types provided they have the same alleles at the major photoperiod and vernalization response loci.
Schematic presentation, using the fine and coarse adjustment knob of the light microscope, of the role of Eps genes in flowering time. The coarse adjustment knob represents the role of photoperiod (Ppd) and vernalization (Vrn) genes in influencing mega environment adaptation while the Eps genes adapt flowering within mega environments. Understanding Eps genes will enable their manipulation and fine-tuning of flowering time which may enable precision breeding in wheat
Earliness per se causes differences of a few days in flowering time under field conditions (Valarik et al. 2006; Griffiths et al. 2009; Zikhali et al. 2014). In Triticcum monococcum, it has been shown that while the Eps effect on chromosome 1A designated Eps-A m 1 causes flowering differences of only a few days at 23 °C, this difference increased to several weeks when the plants were fully vernalized and grown under long days at 16 °C (Appendino and Slafer 2003). In a recent study, Zikhali et al. (2014) it was shown that cultivar Rialto flowers more than 12 days earlier than cultivar Spark in Short days but when grown for 8 weeks under short days and then moved to long days, the Eps effect on chromosome 1DL causes Spark to flower 5 days earlier. This result shows that while the overall difference in flowering time is 5 days, the Eps effect in Spark also overcomes the earliness conferred on Rialto by the short days prior to moving into the long days. Earliness per se is often considered polygenic (Rousset et al. 2011). Determining the role played by the individual Eps genes in each developmental phase may enable breeders to fine tune ear emergence in predictive wheat breeding (Griffiths et al. 2009) and increase wheat yield in different environments (Lewis et al. 2008). To determine the role of an individual Eps gene, on different wheat developmental phases requires knowing what the gene is and hence the need for accurate mapping of the gene responsible (Lewis et al. 2008).
Because of their relatively small effect, Eps genes were previously mapped only as QTLs in wheat (Miura et al. 1999). However, Eps genes have been defined more accurately in the recent years using near isogenic lines (NILs). One Eps gene that has been well defined after almost a decade of study is the Eps-A m 1 reported to be on the distal region of T. monococcum chromosome 1AmL (Bullrich et al. 2002; Valarik et al. 2006; Faricelli et al. 2010). The gene has been recently reported to be involved in determining the number of spikelets as well as the number of grains per spike in diploid wheat in addition to affecting heading time (Lewis et al. 2008). The genes MOLYBDENUM TRANSPORTER 1 (MOT1) and FILAMENTATION TEMPERATURE SENSITIVE H (FtsH4) are the suggested candidates for the Eps-Am1 (Faricelli et al. 2010) although work is in progress to definitively identify the gene responsible. The Eps-3Am locus has also been well defined (Mizuno et al. 2012; Gawroński et al. 2014). The Eps-3Am QTL interval in T. monococcum was fine mapped using high-density mapping (Gawrosnski and Schnurbusch 2012). A recent report suggested a T. monococcum ortholog of the Arabidopsis thaliana LUX ARRHYTHMO/PHYTOCLOCK 1 (LUX/PCL1) as a potential candidate of the Eps-3Am which was suggested to act by distorting the circadian clock (Gawroński et al. 2014).
There are some striking similarities between Eps-Am1 and Eps-3Am. Both Eps-Am1 and Eps-3Am loci were reported to determine the number of spikelets as well as the number of grains per spike in addition to affecting heading time (Lewis et al. 2008; Gawroński et al. 2014). Again both Eps-Am1 and Eps-3Am have been reported to be thermosensitive (Bullrich et al. 2002; Gawroński et al. 2014). This means there is a possibility of manipulating Eps genes to increase yield and optimise adaptation. Grain quality can also be improved by manipulating Eps loci given that Herndl et al. (2008) showed that Eps together with the major genes that control vernalization and photoperiod flowering influence grain protein content.
However, presently there is scant information on the identity of Eps genes, and the mechanism of control that these Eps genes employ in hexaploid wheat. For instance, it is not certain whether Eps genes act independently of environmental cues (Cockram et al. 2007; Laurie et al. 2004; Bullrich et al. 2002), although many reports suggest that this is the case (Lewis et al. 2008; Cockram et al. 2007; Bullrich et al. 2002). Appendino and Slafer (2003) showed that Eps genes could respond to temperature. Laurie et al. (2004) underscored the need to study more about Eps genes given that little is known about them despite their immense potential in improving plant breeding. This was alluded to by Cockram et al. (2007) who suggested that Eps genes were a potential source of variation in targeted breeding given that they were present in both winter and spring crops. The Hordeum vulgare EPS2 locus on chromosome 2H (Laurie et al. 1995) was also reported to be orthologous with the wheat group 2 loci (Laurie 1997). The candidate gene for this locus has only been recently shown in barley to be a homolog of the Antirrrhunum gene CENRORADIALIS (CEN) designated HvCEN (Comadran et al. 2012). Mutations at this gene were shown to cause the wild type indeterminate inflorescence of Antirrhunum to terminate into a flower (Bradley et al. 1996). Analysis of the HvCEN alleles led to the conclusion that HvCEN was important for geographic range extension as well as influencing the gradual separation between spring and winter barley (Comadran et al. 2012). The orthologue of this gene is yet to be identified in the economically important hexaploid wheat.
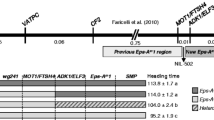
Following the work done by Griffiths et al. (2009), Zikhali et al. (2014) reported the validation of an Eps effect on 1DL in hexaploid wheat (Fig. 39.2). Near isogenic lines (NILs) of a cross between wheat varieties Spark and Rialto grown in the field and controlled environments enabled the QTL on 1DL to be defined as an Eps effect (Zikhali et al. 2014). The NILs segregated for heading date both in short and long days (Fig. 39.2) when fully vernalized (Zikhali et al. 2014). Zikhali et al. (2014) reported that Triticum aestivum FLOWERING LOCUS T 3 (TaFT3) was not a candidate for the 1DL Eps effect. The 1DL Eps locus was reported to be most likely an orthologue of Eps-Am1 and the genes MOT1 and FtsH4 were suggested as possible candidates for 1DL. In addition to MOT1 and FtsH4, the gene T. aestivum EARLY FLOWERING 3 (TaELF3), a circadian clock gene, was also suggested as a possible candidate for 1DL given that it also falls in the QTL interval of 1DL (Zikhali et al. 2014).
Zadoks growth stage 55 for leading tillers of Spark X Rialto NILs grown under controlled environments. The heading days are the mean of 24 plants for the Spark (A) NIL and 30 plants for the Rialto (B) NIL. The additive effect is about five days in the three photoperiod treatments. Student’s t-test was carried out for the mean heading days and all the four NILs pairs have a p value <0.0001, which is highly significant. The error bars are the Standard error of the mean (Adapted from Zikhali et al. (2014))
In a nutshell, Eps genes are gradually being understood with some QTL loci already cloned like the Eps-3Am locus in T. monococum. It is also becoming apparent that Eps genes may not be independent of environmentally cues as previously understood. For example the Eps-3Am locus has been found to have a circadian clock effect, which suggests that this gene responds to photoperiodic changes (Gawroński et al. 2014). Again the thermo sensitivity of both the Eps-Am1 and Eps-3A loci (Gawroński et al. 2014; Bullrich et al. 2002) further suggests that Eps genes are not independent of environmental cues. A more accommodating definition of Eps would be the variation that is observed in flowering time when both vernalization and photoperiod requirements are fully met without being necessarily independent of environmental cues. The additive effect from multiple Eps loci maybe important for wheat adaptation and fine-tuning flowering time.
References
Appendino ML, Slafer GA (2003) Earliness per se and its dependence upon temperature in diploid wheat lines differing in the major gene Eps-A m 1 alleles. J Agri Sci 141:149–154
Appendino ML, Bartoloni N, Slafer GA (2003) Vernalization response and earliness per se in cultivars representing different eras of wheat breeding in Argentina. Euphytica 130:61–69
Beales J, Turner A, Griffiths S et al (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet 115:721–733
Bradley D, Carpenter R, Copsey L et al (1996) Control of inflorescence architecture in Antirrhinum. Nature 379:791–797
Bullrich L, Appendino ML, Tranquilli G et al (2002) Mapping a thermo-sensitive earliness per se gene on Triticum monococcum chromosome 1Am. Theor Appl Genet 105:585–593
Cockram J, Jones H, Leigh FJ et al (2007) Control of flowering time in temperate cereals: genes, domestication, and sustainable productivity. J Exp Bot 58:1231–1244
Comadran J, Kilian B, Russel J et al (2012) Natural variation in a homologue of Antirrhinum CENRORADIALIS contributed to spring growth habit and environmental adaptation in cultivated barley. Nat Genet 44:1388–1392
Dı’az A, Zikhali M, Turner AS et al (2012) Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS One 7:e33234
Distelfeld A, Dubcovsky J (2010) Characterization of the maintained vegetative phase deletions from diploid wheat and their effect on VRN2 and FT transcript levels. Mol Genet Genomics 283:223–232
Distelfeld A, Tranquilli G, Li C et al (2009a) Genetic and molecular variation of the VRN2 loci in tetraploid wheat. Plant Physiol 149:245–257
Distelfeld A, Li C, Dubcovsky J (2009b) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12:178–184
Dixon J, Braun HJ, Kosina P, Crouch J (2009) Wheat facts and futures 2009. CIMMYT, Mexico
Faricelli ME, Valarik M, Dubcovsky J (2010) Control of flowering time and spike development in cereals: the earliness per se Eps-1 region in wheat, rice, and Brachypodium. Funct Integr Genomics 10:293–306
Fu D, Szucs P, Yan L et al (2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Genet Genomics 273:54–65
Gawroński P, Ariyadasa R, Himmelbach A et al (2014) A distorted circadian clock causes early flowering and temperature-dependent variation in spike development in the Eps-3A m mutant of einkorn wheat. Genetics 196:1253–1261
Gawrosnski P, Schnurbusch T (2012) High-density mapping of the earliness per se-3A m (Eps-3A m) locus in diploid einkorn wheat and its relation to the synthetic regions in rice and Brachypodium distachyon L. Mol Breed 30:1097–1108
Gill BS, Appels R, Botha-Oberholster AM et al (2004) A workshop report on wheat genome sequencing: international genome research on wheat consortium. Genetics 168:1087–1096
Greenup A, Peacock WJ, Dennis ES, Trevaskis B (2009) The molecular biology of seasonal flowering in Arabidopsis and cereals. Ann Bot 103:1165–1172
Griffiths S, Simmonds J, Leverington M et al (2009) Meta-QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theor Appl Genet 119:383–395
Herndl M, White JW, Hunt LA et al (2008) Field-based evaluation of vernalization requirement, photoperiod response and earliness per se in bread wheat (Triticum aestivum L). Field Crops Res 105:193–201
Laurie DA (1997) Comparative genetics of flowering time. Plant Mol Biol 35:167–177
Laurie DA, Pratchett N, Bezant JH, Snape JW (1995) RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter x spring barley Hordeum vulgare L. cross. Genome 38:575–585
Laurie DA, Griffiths S, Dunford RP et al (2004) Comparative genetic approaches to the identification of flowering time genes in temperate cereals. Field Crops Res 90:87–99
Lewis S, Faricelli ME, Appendino ML et al (2008) The chromosome region including the earliness per se locus Eps-Am1 affects the duration of early developmental phases and spikelet number in diploid wheat. J Exp Bot 59:3593–3607
Lopes M, Reynolds MP, Manes Y et al (2012) Genetic yield gains and changes in associated traits of CIMMYT spring bread wheat in a ‘Historic’ set representing 30 years of breeding. Crop Sci 52:1123–1131
Miura H, Nakagawa M, Worland AJ (1999) Control of ear emergence time by chromosome 3A of wheat. Plant Breed 118:85–87
Mizuno N, Nitta M, Sato K, Nasuda S (2012) A wheat homologue of PHYTOCLOCK 1 is a candidate gene conferring the early heading phenotype to enkorn wheat. Genes Genet Syst 87:357–367
Reyolds M, Foulkes J, Furbank R et al (2012) Achieving yield gains in wheat. Plant Cell Environ 35:1799–1823
Rosegrant MW, Agcaoili M (2010) Global food demand, supply, and price prospects to 2010. International Food Policy Research Institute, Washington, DC
Rousset M, Bonnin I, Remoué C et al (2011) Deciphering the genetics of flowering time by an association study on candidate genes in bread wheat (Triticum aestivum L.). Theor Appl Genet 123:907–926
Shimada S, Ogawa T, Kitagawa S, Suzuki T, Ikari C, Shitsukawa N, Abe T, Kawahigashi H, Kikuchi R, Handa H, Murai K (2009) A genetic network of flowering time genes in wheat leaves, in which an APETALA1/FRUITFULL-like gene, VRN1, is upstream of LOWERING LOCUS T. Plant J 58:668–681
Shitsukawa N, Ikari C, Shimada S et al (2007) The einkorn wheat (Triticum monococcum) mutant, maintained vegetative phase, is caused by a deletion in the VRN1 gene. Genes Genet Syst 82:167–170
Trevaskis B, Hemming MN, Dennis ES, Peacock WJ (2007) The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci 12:352–357
Valarik M, Linkiewicz AM, Dubcovsky J (2006) A microcolinearity study at the earliness per se gene Eps-A m 1 region reveals an ancient duplication that preceded the wheat-rice divergence. Theor Appl Genet 112:945–957
Van Beem J, Mohler V, Lukman R et al (2005) Analysis of genetic factors influencing the developmental rate of globally important CIMMYT wheat cultivars. Crop Sci 45:2113–2119
Wilhelm EP, Turner AS, Laurie DA (2009) Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.). Theor Appl Genet 118:285–294
Worland AJ, Appendino ML, Sayers EJ (1994) The distribution in European winter wheats of genes that influence ecoclimatic adaptability whilst determining photoperiodic insensitivity and plant height. Euphytica 80:219–228
Worland AJ, Borner A, Korzun V et al (1998) The influence of photoperiod genes on the adaptability of European winter wheats. Euphytica 100:385–394
Yan L, Loukoianov A, Tranquilli G et al (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci U S A 100:6263–6268
Zikhali M, Leverington-Waite M, Fish L et al (2014) Validation of a 1DL earliness per se (Eps) flowering QTL in bread wheat (Triticum aestivum). Mol Breed 34:1023–2033
Acknowledgments
MZ was funded by John Innes foundation and the Sainsbury Laboratory for a rotation PhD. The work was also funded by the grant BB/E006868/1 from the UK Biotechnology and Biological Sciences Research Council. The work also received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 289842 (ADAPTAWHEAT).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Zikhali, M., Griffiths, S. (2015). The Effect of Earliness per se (Eps) Genes on Flowering Time in Bread Wheat. In: Ogihara, Y., Takumi, S., Handa, H. (eds) Advances in Wheat Genetics: From Genome to Field. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55675-6_39
Download citation
DOI: https://doi.org/10.1007/978-4-431-55675-6_39
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55674-9
Online ISBN: 978-4-431-55675-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)