Abstract
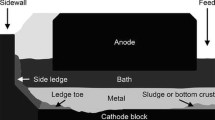
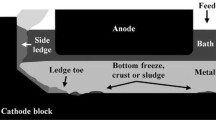
The formation of solid deposits on the cathode surface is problematic for the performance of the electrolysis cell (cathode voltage drop, cell stability, cell life). Furthermore, its mechanism is still not well understood. Although alumina feeding is tightly controlled in industrial cells, the formation of solid deposits is still observed at the interface between the cathode block and aluminum. In order to understand the formation mechanisms and reduce the occurrence of solid deposits, many samples taken from different positions of the interface were analyzed for both the industrial and laboratory electrolysis cells. The effect of the alumina feeding rate and the impact of a thermal gradient on the composition of the deposits were also evaluated. The analysis of industrial and experimental samples demonstrated a concentration gradient of Al2O3 and AlF3 within the deposits implying a variation in the liquidus temperature of the deposit. This study provides insight into the mechanisms responsible for the formation of solid deposits at the cathode surface.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
H. Kvande and W. Haupin, “Cell Voltage in Aluminum Electrolysis: a Practical Approach,” JOM, 52 (2) (2000), 31–37.
X. Wang, “Alumina Dissolution in Aluminum Smelting Electrolyte,” Light Metals, 2009, 383–388.
B. J. Welch and G. I. Kuschel, “Crust and Alumina Powder Dissolution in Aluminum Smelting Electrolytes,” JOM, 59 (5) (2007), 50–54.
R. G. Haverkamp and B. J. Welch, “Modelling the Dissolution of Alumina Powder in Cryolite,” Chemical Engineering and Processing: Process Intensification, 37 (2) (1998), 177–187.
K. Tørklep, K. Kalgraf and T. Nordbø, “Alumina Distribution in Point-Fed Hall-Héroult Cells,” Light Metals, 1997, 377–386.
B. Lillebuen, T. Mellerud and K. Thovsen, “Alumina Dissolution in Point-Fed Cells,” Light Metals, 1992, 449–452.
S. Rolseth, R. Hovland, and O. Kobbeltvedt, “Alumina Agglomeration and Dissolution in Cryolitic Melts,” Light Metals, 1994, 351–357.
R. Keller, “Alumina Dissolution and Sludge Formation Revisited,” Light Metals, 2005, 147–150.
P. Y. Geay, B. J. Welch and P. Homsi, “Sludge in Operating Aluminium Smelting Cells,” Light Metals, 2001, 541–547.
J. Thonstad, S. Ronning and P. Entner, “Formation of Bottom Crusts in Aluminum Pots — a Laboratory Study,” Light Metals, 1982, 485–497.
T. Westphal T. Füllmann and H. Pöllmann, “Rietveld Quantification of Amorphous Portions with an Internal Standard Mathematical Consequences of the Experimental Approach,” Powder Diffraction, 24 (3) (2009), 239–243.
P.-Y. Brisson et al., “Revisiting Sodium and Bath Penetration in the Carbon Lining of Aluminum Electrolysis Cell,” Light Metals, 2005, 727–732.
J. Thonstad et al., Aluminium Electrolysis: Fundamentals of the Hall-Héroult Process (Düsseldorf, Germany: Aluminium-Verlag, 2001), 96.
K. Kalgraf and K. Tørklep, “Sediment Transport and Dissolution in Hall-Héroult Cells,” Light Metals, 1998, 455–464.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 TMS (The Minerals, Metals & Materials Society)
About this chapter
Cite this chapter
Allard, F., Coulombe, MA., Soucy, G., Rivoaland, L. (2014). Cartography and Chemical Composition of the Different Deposits in the Hall-Heroult Process. In: Grandfield, J. (eds) Light Metals 2014. Springer, Cham. https://doi.org/10.1007/978-3-319-48144-9_206
Download citation
DOI: https://doi.org/10.1007/978-3-319-48144-9_206
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48590-4
Online ISBN: 978-3-319-48144-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)