Abstract
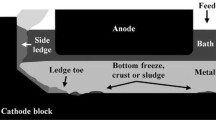
The efficiency of the Hall-Héroult process is influenced by the formation of solid or solid–liquid deposits on the cathode block surface. These deposits have a high electrical and thermal resistivity. Hence, they disturb the electrical current path at the aluminum and cathode block interface. The phase diagrams of deposits with a typical composition were calculated by thermodynamic equilibrium, and the results were confirmed by differential scanning calorimetry measurements. The ledge toe is mainly composed by Na3AlF6(ss), Al2O3(s), and liquid bath in industrial cells. The liquid fraction in the deposits at temperature lower than 933 °C is due to the presence of Na5Al3F14, Na2Ca3Al2F14, and NaCaAlF6 and depends on [Al2O3], [CaF2] and the cryolite ratio (CR). The side ledge in industrial cell contains liquid phase when the CR is lower than 2.8. The sludge contains Al2O3(s) and liquid bath. The ledge toe grows when the local temperature is lower than the solidification temperature of Na3AlF6.












Similar content being viewed by others
References
Sørlie M, Øye HA. Cathodes in aluminium electrolysis. 3rd ed. Düsseldorf: Aluminium-Verlag; 2010.
Chartrand P, Pelton AD. A predictive thermodynamic model for the Al-NaF-AlF3-CaF2-Al2O3 system. Light Met 2002, Proc Int Symp. 2002;245–52.
Apisarov A, Dedyukhin A, Nikolaeva E, Tinghaev P, Tkacheva O, Redkin A, Zaikov Y. Liquidus temperatures of cryolite melts with low cryolite ratio. Metall Mater Trans B. 2011;42:236–42.
Solheim A, Rolseth S, Skybakmoen E, Støen L, Sterten Å, Støre T. Liquidus temperatures for primary crystallization of cryolite in molten salt systems of interest for aluminum electrolysis. Metall Mater Trans B. 1996;27:739–44.
Tissot P. DTA determination of liquidus temperatures and Al2O3 and AlF3 content in cryolitic melts. Thermochim Acta. 1994;234:245–54.
Peterson RD, Tabereaux AT. Liquidus curves for the cryolite—AlF3–CaF2–Al2O3 system in aluminum cell electrolytes. Light Met 1987, Proc Int Symp. 1987;383–8.
Danielik V, Gabčová J. Phase diagram of the system NaF–KF–AlF3. J Therm Anal Calorim. 2004;76:763–73.
Skybakmoen E, Solheim A, Sterten Å. Alumina solubility in molten salt systems of interest for aluminum electrolysis and related phase diagram data. Metall Mater Trans B. 1997;28:81–6.
Coursol P, Dufour G, Côté J, Chartrand P, Mackey P. Application of thermodynamic models for better understanding and optimizing the Hall-Heroult process. JOM. 2012;64:1326–33.
Allard F, Coulombe MA, Soucy G, Rivoaland L. Cartography and chemical composition of the different deposits in the Hall-Heroult process. Light Met 2014, Proc Int Symp. 2014;1233–8.
Thonstad J, Ronning S, Entner P. Formation of bottom crusts in aluminum pots—a laboratory study. Light Met 1982, Proc Int Symp. 1982;485–97.
Keller R. Alumina dissolution and sludge formation revisited. Light Met 2005, Proc Int Symp. 2005;147–150.
Geay PY, Welch BJ, Homsi P. Sludge in operating aluminium smelting cells. Light Met 2001, Proc Int Symp. 2001;2:541–7.
Solheim A, Stoen L. On the composition of solid deposits frozen out from cryolitic melts. Light Met 1997, Proc Int Symp. 1997;325–32.
Solheim A. Some aspects of heat transfer between bath and sideledge in aluminium reduction cells. Light Met 2011, Proc Int Symp. 2011;381–6.
Fallah-Mehrjardi A, Hayes PC, Jak E. Investigation of freeze-linings in aluminum production cells. Metall Mater Trans B. 2014;. doi:10.1007/s11663-014-0078-z.
Allard F, Soucy G, Rivoaland L. Formation of deposits on the cathode surface of aluminum electrolysis cells. Metall Mater Trans B. 2014;. doi:10.1007/s11663-014-0118-8.
Lukas K, LeMaire PK. Differential scanning calorimetry: fundamental overview. Resonance. 2009;14:807–17.
Haines PJ. Thermal methods of analysis: principles, applications and problems. 1st ed. London: Chapman & Hall; 1995.
Cuesta D, Gόmez MA, Porteiro J, Febrero L, Granada E, Arce E. CFD analysis of a TG–DSC apparatus. J Therm Anal Calorim. 2014;. doi:10.1007/s10973-014-3734-2.
Zaitseva JN, Yakimov IS, Kirik SD. Thermal transformation of quaternary compounds in NaF–CaF2–AlF3 system. J Solid State Chem. 2009;182:2246–51.
Feret FR. Breakthrough in analysis of electrolytic bath using Rietveld-XRD method. Light Met 2008, Proc Int Symp. 2008;343–6.
Craig DF, Brown JJ. Phase equilibria in the system CaF2–AlF3–Na3AlF6 and part of the system CaF2–AlF3–Na3AlF6–Al2O3. J Am Ceram Soc. 1980;63:254–61.
Holm JL. The phase diagram of the system Na3AlF6–CaF2, and the constitution of the melt in the system. Acta Chem Scand. 1968;22:1004–12.
Taylor MP, Liu X, Fraser KJ, Welch BJ. Dynamics and performance of reduction cell electrolytes. Light Met 1990, Proc Int Symp. 1990;259–66.
Howard EH. Some physical and chemical properties of a new sodium aluminum fluoride. J Am Chem Soc. 1954;76:2041–2.
Kvande H. Vapour-phase studies of NaF–AlF3 melts. 1. The AlF3–rich part. High Temp High Press. 1983;15:51–62.
Acknowledgements
The authors are grateful to the employees of the “Centre de Caractérisation des Matériaux” for their help with the various characterization devices and to the chemist, Mr. Carl St-Louis, who helped to elaborate the DSC method. The authors also thank Alireza Hekmat and Lynne Davies for manuscript revision and correction. This work is financed and supported by Rio Tinto Alcan, “Conseil de Recherches en Sciences Naturelles et en Génie du Canada” (CRSNG) and “Fonds de Recherche du Québec-Nature et Technologies” (FRQNT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allard, F., Soucy, G., Rivoaland, L. et al. Thermodynamic and thermochemical investigation of the deposits formed on the cathode surface of aluminum electrolysis cells. J Therm Anal Calorim 119, 1303–1314 (2015). https://doi.org/10.1007/s10973-014-4288-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4288-z