Abstract
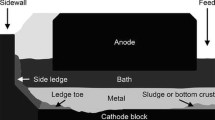
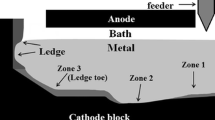
The efficiency of electrolysis cells for aluminum production is reduced when deposits are formed on the cathode block surface. Overfeeding of alumina or excessive heat loss in industrial cells leads to the formation of highly resistive deposits. In this study, the chemical composition of sludge, ledge toe, and thin deposits was investigated at the bottom of both industrial and experimental electrolysis cells. The formation of deposits in laboratory experiments was demonstrated in acidic, neutral, and basic electrolytic bath. A gradient of chiolite (Na5Al3F14) and α-Al2O3 was observed in the deposits. The bath at the bottom of the experimental electrolysis cell had a higher cryolite ratio implying a higher liquidus temperature. The sludge formed at the bottom of the cell can lift the aluminum metal resulting in an important reduction of the contact surface between the aluminum and the cathode block. Moreover, the deposits disturb the current path and generate horizontal current components in the metal which enhance the motion and lower the current efficiency. A thin film of bath supersaturated in alumina was observed under the metal. This work provides clarification on the formation mechanisms of the various deposits responsible for the deterioration of the cathode surface.








Similar content being viewed by others
References
H. Kvande, B.P. Moxnes, J. Skaar, and P.A. Solli: Light Met. 1997, Proc. Int. Symp., 1997, pp. 403–09.
M. Sørlie, and H.A. Øye: Cathodes in Aluminium Electrolysis, 3rd ed., Aluminium-Verlag, Düsseldorf, Germany, 2010, pp. 165.
J. Thonstad, P. Johansen, and E.W. Kristensen: Light Met. 1980, Proc. Int. Symp., 1980, pp. 227–39.
J. Thonstad, S. Rønning, and P. Entner: Light Met. 1982, Proc. Int. Symp., 1982, pp. 485–97.
L.N. Less: Metall. Trans. B, 1977, vol. 8B, pp. 219-25.
R. Keller: Light Met. 1984, Proc. Int. Symp., 1984, pp. 513–18.
S. Rolseth, R. Hovland, and O. Kobbeltvedt: Light Met. 1994, Proc. Int. Symp., 1994, pp. 351–57.
X. Liu, S.F. George, and V.A. Wills: Light Met. 1994, Proc. Int. Symp., 1994, pp. 359–64.
N.P. Østbø: Ph.D. Dissertation, Norwegian University of Science and Technology, Trondheim, Norway, 2002.
P.Y. Geay, B.J. Welch, and P. Homsi: Light Met. 2001, Proc. Int. Symp., 2001, pp. 541–47.
K. Tørklep, K. Kalgraf, and T. Nordbø: Light Met. 1997, Proc. Int. Symp., 1997, pp. 377–86.
R.G. Haverkamp, and B.J. Welch: Chem. Eng. Process., 1998, vol. 37, pp. 177-87.
B.J. Welch, and G.I. Kuschel: JOM, 2007, vol. 59, pp. 50-54.
X. Wang: Light Met. 2009, Proc. Int. Symp., 2009, pp. 383–88.
R. Keller: Light Met. 2005, Proc. Int. Symp., 2005, pp. 147–50.
F. Allard, M.A. Coulombe, G. Soucy, and L. Rivoaland: Light Met. 2014, Proc. Int. Symp., 2014, pp. 1233–38.
F.R. Feret: Light Met. 2008, Proc. Int. Symp., 2008, pp. 343–46.
A. Solheim: Light Met. 2002, Proc. Int. Symp., 2002, pp. 225–30.
P.Y. Brisson, H. Darmstadt, M. Fafard, A. Adnot, G. Servant, and G. Soucy: Carbon, 2006, vol. 44, pp. 1438-47.
A. Solheim, S. Rolseth, E. Skybakmoen, L. Støen, Â. Sterten, and T. Støre: Metall. Mater. Trans. B, 1996, vol. 27B, pp. 739-44.
T.A. Utigard: Light Met. 1999, Proc. Int. Symp., 1999, pp. 319–26.
J. Thonstad: Aluminium Electrolysis: Fundamentals of the Hall-Héroult Process, 3rd ed. Aluminium-Verlag, Düsseldorf, Germany, 2001.
R. Ødegård, Å. Sterten, and J. Thonstad: Metall. Trans. B, 1988, vol. 19B, pp. 449-57.
R.G. Munro: J. Am. Ceram. Soc., 1997, vol. 80, pp. 1919-28.
G.J. Janz: J. Phys. Chem. Ref. Data, 1988, vol. 17, pp 1-325.
K. Kalgraf, and K. Tørklep: Light Met. 1998, Proc. Int. Symp., 1998, pp. 455–64.
Acknowledgments
The authors are grateful to the employees of the “Centre de Caractérisation des Matériaux” for their help with the various characterization devices and to the mechanical engineering technician, André Bilodeau, who helped with the design of the experimental equipment. The help of Marc-André Coulombe for the experimental tests and analysis is greatly acknowledged. The authors also thank Alireza Hekmat for manuscript revision and correction. This work is financed and supported by Rio Tinto Alcan, “Conseil de Recherches en Sciences Naturelles et en Génie du Canada” (CRSNG) and “Fonds Québécois de la Recherche sur la Nature et les Technologies” (FQRNT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 10, 2014.
Rights and permissions
About this article
Cite this article
Allard, F., Soucy, G. & Rivoaland, L. Formation of Deposits on the Cathode Surface of Aluminum Electrolysis Cells. Metall Mater Trans B 45, 2475–2485 (2014). https://doi.org/10.1007/s11663-014-0118-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0118-8