Abstract
The association between subjective memory complaints (SMCs) and depressive symptoms has been widely reported and both have been regarded as risk factors for dementia, such as Alzheimer’s disease (AD). Although SMCs arise as early as in middle age, the exact neural correlates of comorbid depressive symptoms among individuals who are middle-aged and with SMCs have not yet been well investigated. Because rich-club organization of the brain plays a key role in the pathophysiology of various neuropsychiatric disorders, the investigation of rich club organization may provide insight regarding the neurobiological mechanisms of depressive symptoms in SMCs. In the current study, we compared the rich-club organization in the structural brain connectivity between individuals who have SMCs along with depressive symptoms (SMCD) and individuals with SMCs but without depressive symptoms (SMCO). A total of 53 individuals with SMCD and 91 individuals with SMCO participated in the study. For all participants, high-resolution, T1-weighted images and diffusion tensor images were obtained, and the network analysis was performed. Individuals with SMCD had lower connectivity strength between the precuneus and other rich-club nodes than those with SMCO, which was significant after adjusting for potential confounders. Our findings suggest that disruptions of rich-club connectivity strength of the precuenus are associated with depressive symptoms in middle-aged individuals with SMCs. Given that the precuneus is one of the commonly affected regions in the early stages of AD, our findings may imply that the concomitant depressive symptoms in middle-aged individuals with SMCs could reflect structural alterations related to AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subjective memory complaints (SMCs) have been characterized as subjective reports of cognitive and memory problems without objective deficits in the cognitive domain as shown in mild cognitive impairment (MCI) or dementia (Hafkemeijer et al., 2013; Yasuno et al., 2015). SMCs, which may occur before the clinical diagnosis of MCI, have received increasing attention with respect to their implications for the early detection of dementia (Scheef et al., 2012; Stewart et al., 2011). For instance, various research in the past, including both clinical and neuroimaging studies, have suggested that SMCs are considered as one of the most frequently observed symptoms in the early stages of dementia (Juncos-Rabadan et al., 2012; Reisberg & Gauthier, 2008; Stewart et al., 2008; Stewart et al., 2011; Sun et al., 2016; Waldorff, Siersma, Vogel, & Waldemar, 2012; Yasuno et al., 2015). In addition, some studies have found that SMCs are associated with an increased risk of developing Alzheimer’s disease (AD) (Geerlings, Jonker, Bouter, Adèr, & Schmand, 1999; Jessen et al., 2010) and progressive cognitive impairment (Buckley R. F. et al., 2016; Lista et al., 2015).
Because depressive symptoms are frequently observed in subjects with SMCs (Hohman, Beason-Held, & Resnick, 2011; Montejo et al., 2014; Sousa, Pereira, & Costa, 2015), previous studies have suggested that SMCs may constitute symptom constellations of depression (Balash et al., 2013; Balash, Mordechovich, Shabtai, Merims, & Giladi, 2010; Buckley et al., 2013; Chin, Oh, Seo, & Na, 2014; Zlatar, Moore, Palmer, Thompson, & Jeste, 2014). However, given that depressive symptoms also are known to be a risk factor for developing cognitive impairment and dementia, and the severity of depressive symptoms in older adults are well correlated with the burden of AD pathology (Lavretsky et al., 2009), the comorbidity of depressive symptoms in subjects with SMCs may play a significant role in the potential progression from SMCs to dementia.
As mentioned above, the association between depressive symptoms and SMCs has been one of the most robust findings in scientific research (Brigola et al., 2015; Lehrner et al., 2014; Montejo Carrasco et al., 2017; Sousa et al., 2015; Zlatar, Muniz, Galasko, & Salmon, 2017). However, the neural correlates of the comorbidity between depressive symptoms and SMCs have not yet been elucidated. Previous neuroimaging studies have shown that depressive symptoms in nondemented elderly are associated structural brain abnormalities, especially in AD-related structures, such as limbic structures (Dotson, Davatzikos, Kraut, & Resnick, 2009; Szymkowicz et al., 2018; Zhou et al., 2016). Thus, concomitant depressive symptoms in the presence of SMCs may be the result of early structural alterations of the brain while in progression towards AD, rather than a symptom in itself or one of the reflective features related to SMCs. Therefore, understandings of the neural mechanisms underlying the comorbidity of depressive symptoms in SMCs may provide important insights into the early diagnosis and intervention for individuals at risk for dementia.
Furthermore, recent research has shown that SMCs are associated with the onset of cognitive impairment that occurs approximately two decades subsequent to the SMCs (Kaup, Nettiksimmons, LeBlanc, & Yaffe, 2015), which suggests that SMCs may be a very early symptom of an insidious neurodegenerative diseases, such as AD. In addition, individuals with SMCs as early as in middle-age have shown significant cortical thinning in several brain regions that are commonly affected in AD, including the entorhinal, posterior cingulate, and inferior parietal cortices, compared with healthy control subjects (Schultz et al., 2015). However, most previous studies related to SMCs and depression have primarily focused on the elderly (Brigola et al., 2015; Montejo Carrasco et al., 2017). Given that SMCs can arise as early as middle-age, along with the fact that management of early risk factors may mitigate further cognitive impairment (Byers & Yaffe, 2011; Hughes & Ganguli, 2009), it would be more informative and beneficial to investigate the neural mechanisms of depressive symptoms in middle-aged individuals with SMCs rather than in the elderly.
The human brain is organized into a large-scale network (Gong et al., 2009; Hagmann et al., 2008; Hagmann et al., 2007), which is characterized by an optimal balance between the integration and segregation of information (Latora & Marchiori, 2001). Recent neuroimaging research have found that several hub regions, such as the superior frontal, superior parietal, insula, and precuneus in the human brain, play an important role in the interregional communication and hierarchical organization of the brain (van den Heuvel & Sporns, 2011; van den Heuvel et al., 2013; Yoon et al., 2016). These network hubs have been known to form a central core otherwise known as rich-club that is highly interconnected with each other. Notably, rich-club brain regions, which also are referred to as network hubs, have been involved in global integration of information (van den Heuvel & Sporns, 2011; van den Heuvel et al., 2013). Previous studies have suggested that disruptions in rich-club brain regions may contribute to the pathophysiology of several neuropsychiatric and neurodegenerative diseases including schizophrenia, depression, and AD (Fischer, Wolf, Scheurich, Fellgiebel, & Alzheimer's Disease Neuroimaging Initiative, 2015; van den Heuvel et al., 2013; Yoon et al., 2016). Because alterations in rich-club organization may occur before the occurrence of any conspicuous symptoms related to a disease (McColgan et al., 2015), the investigation of rich-club organizations may provide structural network-based information that is relevant to the early stage of disease.
To identify the neurobiological mechanism of comorbid depressive symptoms of SMCs in a sample of middle-aged adults, the current study investigated whether there were differences in rich-club organization in the structural brain network between individuals with SMCs who had coexisting depressive symptoms (SMCD) and individuals with SMCs but without depressive symptoms (SMCO). In the case that depressive symptoms are merely one of many symptoms related to SMCs, one might expect that there are no differences in the structural brain network between the SMCD and SMCO groups. However, under the assumption that depressive symptoms in SMCs may be a marker for disruptions in the structural brain network, there may be between-group differences in alterations of the structural brain network that can be explained by the level of depressive symptoms. Therefore, we hypothesized that the structural disruptions of rich-club connectivity are associated with the manifestation of depressive symptoms in individuals with SMCs.
Materials and methods
Participants
Initially, a total of 150 participants who had complaints about their memory or with cognitive difficulty were recruited. The participants were administered the Subjective Memory Complaints Questionnaire (SMCQ) (Youn et al., 2009) to determine whether they had SMCs. Participants who answered “yes” to 1 or more of the 14 questions of the SMCQ were considered as having SMCs, whereas those who answered “no” to all 14 questions were regarded as not having SMCs. Based on this criterion, 6 participants answered “no” to all 14 questions of the SMCQ and therefore were excluded from the study. A total of 144 participants were finally enrolled in the study.
All participants met the following criteria: 1) aged between 45 and 65 years; 2) years of education ≥6; 3) the scores of the Korean version of the Mini Mental Status Examination (MMSE-K) (Park, 1989) ≥26; and 4) the scores of the Korean version of Clinical Dementia Rating (CDR-K) (Choi et al., 2001) = 0. We excluded individuals with any of the following: 1) suspected or diagnosed with mild cognitive impairment or dementia; 2) suspected or diagnosed with any major neurological or psychiatric illnesses, including major depressive disorder (MDD); 3) any contraindications to magnetic resonance imaging (MRI); 4) visual or hearing impairments severe enough to interfere with questionnaire response; 5) a history of medications that could affect cognitive and emotional functions in the past 3 months; or 6) any other major medical problems. All of the participants were interviewed by two board-certified psychiatrists using the CDR-K and the Korean version of the Structured Clinical Interview for DSM-IV (SCID-IV-K) (Hahn et al., 2000). The CDR scale is a widely accepted structured interview in which subjects are rated as having scores of 0 (asymptomatic), 0.5 (equivocal or mild impairment), 1 (mild), 2 (moderate), or 3 (severe dementia) (Choi et al., 2001; Morris, 1997).
Written, informed consent was obtained from all participants, and the study protocol was approved by the Institutional Review Board of Ewha W. University.
Clinical assessments
Participants with depressive symptoms were previously defined as those who have subclinical depression (Hayakawa et al., 2013; Lavretsky & Kumar, 2002). In the current study, as in other previous studies, the term subclinical depressive symptoms refers to having significant depressive symptoms, which is based on the cut-off score of 14 on the Korean version of Beck Depression Inventory-II (BDI-II-K) (Sung et al., 2008), while not meeting the DSM-IV diagnostic criteria for MDD. The cutoff score of 14 on the BDI-II-K was used to classify participants with SMCs into two groups: individuals with SMCs who had depressive symptoms (n = 53, hereafter referred to as the SMCD group) and those with SMCs who did not have depressive symptoms (n = 91, hereafter referred to as the SMCO group).
Previous studies have shown that individuals with SMCs have commonly reported memory problems (Cook & Marsiske, 2006; Jessen et al., 2007; Kurt, Yener, & Oguz, 2011). Moreover, deficits in visual and working memory functions have also been frequently observed in individuals with depression (Christopher & MacDonald, 2005; Hammar et al., 2011; Kalska et al., 1999; Porter et al., 2003). Thus, visual and working memory domains were the primary choice of assessment in both SMCD and SMCO groups using the following three cognitive tasks implemented in the Korean version of Cambridge Neuropsychological Test Automated Battery (CANTAB) (Kim et al., 2009; Kim & Cho, 2012; Kim et al., 2014; Sharma, 2013). Visual memory performance was measured using the Spatial Recognition Memory (SRM) task and Delayed Matching to Sample (DMS) task, whereas working memory was evaluated using the Spatial Working Memory (SWM) task. The SRM task has a two-forced-choice discrimination paradigm that evaluates spatial recognition memory (Owen et al., 1995). The outcome of the SRM task includes the percentage of correct responses. During the DMS task, the participants were asked to recall the visual components of a complex, abstract target stimulus provided, and then identify it from the four target patterns provided after a delay (Robbins et al., 1994). The outcome measure of the DMS task includes the percentage of correct responses. Finally, the SWM task is a self-ordered search task, which evaluates working memory. Participants were instructed to search for tokens without returning to previous token locations (Owen et al., 1995). The outcome measure of the SWM is total number of errors.
Each raw score of the above mentioned three tasks was converted to a standardized Z score. Among the three tasks, as the SWM raw scores indicated the total number of errors, the scores of SWM were reversed such that positive Z scores represent better performance. The composite score for the visual memory domain was constructed by averaging Z scores of the correct responses in the SRM and DMS tasks. The standardized Z score of the total errors in the SWM task was used as the representative score for the working memory domain.
Imaging data acquisition
High-resolution T1-weighted and diffusion-weighted images were acquired using a 3.0 Tesla Philips Achieva MR scanner (Philips Medical System, Bests, Netherlands). Magnetization-prepared rapid gradient echo imaging sequence was used to obtain a three-dimensional T1-weighted images with the following acquisition parameters: repetition time (TR) = 7.4 ms, echo time (TE) = 3.4 ms, flip angle (FA) = 8°, field of view (FOV) =220 X 220 mm2, slice thickness = 1 mm, number of excitation (NEX) = 1, 180 contiguous sagittal slices. In addition to b0 image without weighting, diffusion-weighted images were acquired from 32 different directions with the following parameters: b = 1,000 s/m2, TE = 68 ms, TR = shortest, matrix = 96 x 96, FOV = 200 X 200 mm2, flip angle = 90°, NEX = 2, slice thickness = 1.8 mm. Additional axial proton density/T2-weighted images were acquired to evaluate structural abnormalities in the brain with the following parameters: TR = 3,000 ms, TE = 9.5/90 ms, FA = 90°, FOV = 202 X 201 mm2, slice thickness = 2.5 mm, NEX = 1. Acquisition parameters for sagittal fluid-attenuated inversion recovery images were as follows: TR = 8,000 ms, TE = 332 ms, inversion time = 2,400 ms, FA = 90°, FOV = 250 X 250 mm2, slice thickness = 0.6 mm, NEX = 1.
Image processing
T1-weighted image of each participant was parcellated into 34 cortical and 7 subcortical regions per each hemisphere using the FreeSurfer program (http://surfer.nmr.mgh.harvard.edu) (Desikan et al., 2006). A total of 68 cortical and 14 subcortical regions-of-interests (ROIs) was defined as the nodes of the structural brain network (van den Heuvel & Sporns, 2011). A T1-weighted image of each participant was coregistered to the corresponding nondiffusion image (b = 0 s/m2) using affine transformation. All cortical and subcortical ROIs in the native space were inversely transformed to the diffusion space.
Diffusion-weighted images were linearly registered to the non-diffusion image (b = 0 s/m2) in order to correct for head motion and eddy current distortions. Diffusion tensor was fitted and fractional anisotropy (FA) values were computed within each voxel using the Diffusion Toolkit (http://trackvis.org/) (Wang, Beener, Sorensen, & Wedeen, 2007).
Network construction
Detailed information on the network construction is described elsewhere (Yoon et al., 2016). In brief, white matter tracts were reconstructed using the deterministic fiber tracking on the basis of Fiber Assignment by Continuous Tracking (FACT) algorithm (Mori, Crain, Chacko, & van Zijl, 1999).
A streamline was terminated when it reached a voxel with a FA value < 0.1, had a turning angle > 45 degrees, or exceeded the ROI. Through these processes implemented in the Trackvis (http://trackvis.org) software package, all fiber tracts interconnecting 82 ROIs were reconstructed. Streamlines shorter than 10 mm were considered as being spurious and were not included in further analysis.
Fiber tracts interconnecting 68 cortical and 14 subcortical nodes were combined to reconstruct the structural brain network comprising a set of nodes and edges. To reduce the risk of false-positive connections, a threshold of the number of fiber tracts was applied similar to previous studies (Lo et al., 2010; Shu et al., 2011). Specifically, the structural connection of two nodes by an edge was considered as established if there is a minimum of three streamlines that are connected between the two nodes. Fiber numbers determined by streamline tracking may reflect the white matter structure (Houenou et al., 2007) and have been used as a weight for network edges (Batalle et al., 2012; Shu et al., 2011; Yan et al., 2011; Zhang et al., 2011). Finally, the weighted structural networks represented as symmetric 82 x 82 matrices were constructed for each individual.
Assessment of global network metrics
The network analysis was performed using the Brain Connectivity Toolbox (http://www.brain-connectivity-toolbox.net). The connectivity strength, global efficiency (Eglob), local efficiency (Eloc), and clustering coefficient were computed in each matrix to assess graph metrics of the global topological organization of whole-brain structural connectivity network. Connectivity strength is defined as the sum of all the streamlines connecting between nodes (Rubinov & Sporns, 2010). Global efficiency is a measure of network integration and defined as the average inverse shortest path lengths between all pairs of nodes (Latora & Marchiori, 2001), whereas local efficiency is a measure of segregation of structural connectivity network and is defined as the efficiency computed on node neighborhoods (Rubinov & Sporns, 2010; Watts & Strogatz, 1998). To measure network segregation, the weighted clustering coefficient of a node was computed and was defined as the likelihood of the neighborhoods being connected with each other (Onnela, Saramaki, Kertesz, & Kaski, 2005). The mean clustering coefficient of a network was calculated as the average of the clustering coefficient across all nodes.
Assessment of rich-club organization
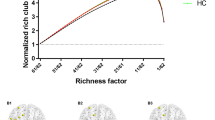
The rich-club nodes were selected according to the guidelines provided in previous studies (van den Heuvel & Sporns, 2011; Yoon et al., 2016). Briefly, because random networks also show increasing network connections due to the fact that nodes with a higher degree also have a higher probability of being interconnected by chance, we computed the normalized rich club coefficient to show the existence of rich club organization in a network. Rich-club coefficient, φ (κ), is typically normalized relative to a set of comparable random networks of equal size and similar connectivity distribution (van den Heuvel & Sporns, 2011). As such, for each network, m = 1,000 random networks were computed by shuffling the links, preserving the weights and the degree sequence and thus all node degrees including the hubs in the network. Like other previous studies (van den Heuvel et al., 2013), a normalized rich-club coefficient φ norm (κ) of greater than 1 over a range of κ is defined as the existence of rich club organization in a network. The presence of rich club organization was identified by performing a two-tailed t-test with the permutation testing (10,000 permutations) of the area under the curve (normalized weighted rich-club coefficient against degree) for each group versus random network. The comparison of rich-club coefficient between the two groups was performed with the general linear model (GLM) after adjusting for age and sex (Fig. S1, see online Supplementary Material ).
We defined rich-club nodes as the top 10 highest ranking nodes (12%) on the basis of the degree of nodes within the group-averaged structural brain network (van den Heuvel et al., 2013; Yoon et al., 2016). The selected rich-club nodes include the superior frontal cortex (SFC), superior parietal cortex (SPC), precuneus, putamen, and thalamus, all bilaterally. Rich-club nodes are likely to be highly connected with each other compared to connections that would be expected by random chance (van den Heuvel & Sporns, 2011). All connections between nodes in each structural matrix were categorized into one of the following categories: “rich-club connections,” which are defined as the number of all connections linking rich-club nodes; “feeder connections,” which are defined as the number of connections linking rich-club to non-rich-club nodes, and “local connections,” which are defined as the number of connections linking between non-rich-club nodes (van den Heuvel & Sporns, 2011).
Statistical analyses
For the baseline demographic data, the independent t-test and the chi-square test were used for the continuous variables and the dichotomous variables, respectively. The group differences in cognitive functions were also assessed using analysis of covariance (ANCOVA) with age, sex, and years of education as covariates.
Global network metrics including global efficiency, local efficiency, and clustering coefficient of the whole-brain structural connectivity network were compared between the SMCD and SMCO groups using the general linear model (GLM) after adjusting for age, sex, and years of education. The GLM also was used to examine differences in rich-club, feeder, and local connections between the SMCD and SMCO groups using age and sex as covariates. Partial eta squared (ηp2) was used to estimate the effect size. Spearman correlation analysis was performed to estimate the associations between clinical characteristics and connectivity strength in rich-club connections in the SMCO and SMCD groups.
Correction for multiple comparisons was performed using a permutation-based test (Hurtz et al., 2014; Nichols & Holmes, 2002). Data were tested against an empirical null distribution by running 10,000 synthesized permutations with a threshold of p < 0.05. (Westfall, Young, & Wright, 1993). All analyses were performed using the STATA 13.0 (StataCorp, College Station, TX).
Results
Basic information
Clinical characteristics
There were no significant differences in age, gender, years of education, and MMSE-K scores between the SMCD and SMCO groups (Table 1). The SMCD group (mean ± SD, 5.57 ± 3.08; median, 5.0; interquartile range, 3.0e, genshowed higher scores on the SMCQ compared with the SMCO group (mean ± SD, 3.52 ± 2.54; median, 3.0; interquartile range, 1.0-5.0) (t = −4.29, P < 0.001).
There were no significant differences in visual memory performance (mean Z score ± SD, 0.09 ± 0.76 vs. −0.20 ± 0.72, P = 0.050) and working memory performance (0.09 ± 0.96 vs. −0.21 ± 1.07, P = 0.151) between the SMCO and SMCD groups (Table 2).
Global network metrics
There were no significant differences in global network topologies, including connectivity strength (mean ± SD. 0.266 ± 0.027 vs. 0.267 ± 0.027, ηp2= 0.000, permutation adjusted P = 0.867), global efficiency (0.016 ± 0.002 vs. 0.015 ± 0.002, ηp2 = 0.004, permutation adjusted P = 0.417), and clustering coefficient (0.007 ± 0.001 vs. 0.007 ± 0.001, ηp2 = 0.000, permutation adjusted P = 0.762) between the SMCO and SMCD groups.
Primary analyses
Rich-club organization
There were no differences in connectivity strength of rich-club connections (ηp2 = 0.017, permutation-adjusted P = 0.122), feeder connections (ηp2 = 0.001, permutation-adjusted P = 0.757), and local connections (ηp2 = 0.003, permutation-adjusted P = 0.529) between the SMCD and SMCO groups (Figure 1). Likewise, there were no significant differences in network efficiency of rich-club connections (ηp2 = 0.012, permutation-adjusted P = 0.197), feeder connections (ηp2 = 0.001, permutation-adjusted P = 0.701), and local connections (ηp2 = 0.001, permutation-adjusted P = 0.790) between the two groups (Figure 1).
The mean connectivity strength and efficiency of rich-club, feeder, and local connections. *Error bars indicate standard error. There were no differences in connectivity strength of rich-club connections (ηp2 = 0.017, permutation-adjusted P = 0.122), feeder connections (ηp2 = 0.001, permutation-adjusted P = 0.757), and local connections (ηp2 = 0.003, permutation-adjusted P = 0.529) between the SMCD and SMCO groups. Likewise, there were no significant differences in network efficiency of rich-club connections (ηp2 = 0.013, permutation-adjusted P = 0.197), feeder connections (ηp2 = 0.001, permutation-adjusted P = 0.701), and local connections (ηp2 = 0.001, permutation-adjusted P = 0.790) between the two groups
To examine region-specific alterations in rich-club connections, we computed the connectivity strength of each rich-club node linking to other rich-club nodes. Rich-club connections of the SFC, SPC, precuneus, putamen, and thalamus were calculated in each hemisphere. Connectivity strength between the precuneus and other rich-club nodes was lower in the SMCD group than in the SMCO group (ηp2 = 0.030, permutation-adjusted P = 0.040) (Figure 2). When we examined the left and the right precuneus, respectively, only the connectivity strength of the left precuneus was significantly reduced in the SMCD group (ηp2 = 0.039, permutation-adjusted P = 0.019) than in the SMCO group, while that of the right precuenus showed no significant group differences (ηp2 = 0.015, permutation-adjusted P = 0.150). To ensure the robustness of our results, we also compared the connectivity strength of the precuneus between the two groups after adjusting for age, sex, years of education, and the composite scores of the visual memory and working memory functions, which also showed significant group differences (ηp2 = 0.030, permutation-adjusted P = 0.041). There were no differences in rich-club connectivity of other rich-club nodes between the groups (SFC, ηp2 = 0.016, permutation-adjusted P = 0.150; SPC, ηp2 = 0.002, permutation-adjusted P = 0.617; thalamus, ηp2 = 0.001, permutation-adjusted P = 0.771; putamen, ηp2 = 0.001, permutation-adjusted P = 0.793).
Rich-club connectivity strength among the rich-club nodes. *Error bars indicate standard error. Further analysis for the region-specific alterations in the rich-club connections revealed that the connectivity strength between the precuneus and other rich-club nodes was lower in the SMCD group than in the SMCO group (ηp2 = 0.030, permutation-adjusted P = 0.041). There were no differences in rich-club connectivity of other rich-club nodes between the groups (superior frontal cortex, ηp2 = 0.016, permutation-adjusted P = 0.150; superior parietal cortex, ηp2 = 0.002, permutation-adjusted P = 0.617; thalamus, ηp2 = 0.001, permutation-adjusted P = 0.771; putamen, ηp2 = 0.001, permutation-adjusted P = 0.793)
Secondary analyses
Relationships between rich-club organization of the precuneus and clinical characteristics
The connectivity strength of rich-club connections in the precuneus was significantly correlated with working memory function (ρ = 0.370, P = 0.006) in the SMCD group (Figure 3). However, there was no correlation between the connectivity strength of the precuneus and working memory function (ρ = −0.055, P = 0.603) in the SMCO group. In addition, there were no relationships between the connectivity strength of rich-club connections in the precuneus and visual memory function in both the SMCO (ρ = −0.132, P = 0.213) and SMCD (ρ = −0.219, P = 0.116) groups. In terms of within group analysis, the connectivity strength of rich-club connections in the precuneus was not correlated with the severity of depressive symptoms in both SMCO (ρ = −0.091, P = 0.391) and SMCD (ρ = −0.040, P = 0.774) groups, respectively.
Correlations between working memory function and rich-club connections of the precuneus between the SMCD and SMCO group. The connectivity strength of rich-club connections in the precuneus was significantly associated with the performance on working memory composite in the SMCD group (ρ = 0.370, P = 0.006) (a), whereas there was no correlation between the connectivity strength of the precuneus and the performance on working memory composite in the SMCO group (ρ = −0.055, P = 0.603) (b)
Exploratory analyses
Connectivity strength between the Hippocampus and other brain regions
The hippocampus was not selected as a rich club region in the current study, based on the definition of rich-club nodes as being the top 10 highest ranking nodes (12 %) on the basis of the degree of nodes within group-averaged structural brain network (van den Heuvel et al., 2013; Yoon et al., 2016). However, as previous studies have indicated, the hippocampus is one of the key brain regions in the field of SMCs and depression (Sawyer et al., 2012; Stewart et al., 2011; von Gunten et al., 2000; von Gunten & Ron, 2004). Therefore, we have performed additional analyses, which investigated the between-group differences in hippocampal connectivity strength with other brain regions to provide more contextual information regarding the hippocampus (Figure 4). There were no significant differences in hippocampal connectivity strength between the SMCO and SMCD groups (mean ± SD. 0.523 ± 0.178 vs. 0.536 ± 0.169, ηp2 = 0.002, permutation adjusted P = 0.627).
Connectivity strength of the precuneus according to the degree of depressive symptoms or the degree of SMCs
We have performed additional sensitivity analyses to investigate how the rich-club connectivity of the precuneus may be predicted by the degree of depressive symptoms or the degree of SMCs (Supplementary Results-2 and Fig. S2, see online Supplementary Material ). The results from these additional analyses show that the rich-club connectivity of the precuneus is associated with the severity of depressive symptoms but not the degree of SMCs.
Discussion
To the best of our knowledge, this is the first study to demonstrate that the connectivity strength of the precuneus was reduced in the SMCD group compared with the SMCO group, which may suggest that structural disruptions in the rich-club connections of the precuneus are associated with the manifestation of depressive symptoms in SMCs.
It has been suggested that the hubs or rich-clubs of the brain may be more vulnerable to structural changes in the brain network due to the higher nodal degree of the hub regions relative to non-rich-club regions (Crossley et al., 2014). Previous studies have shown that rich-club connections were lower in patients with neuropsychiatric diseases, such as MDD (Yoon et al., 2016), schizophrenia (van den Heuvel et al., 2013), and Huntington’s disease (McColgan et al., 2015) compared with healthy controls. In the current study, the reduced connectivity strength of the precuneus in the SMCD group was significant after adjusting for potential confounders, including age, sex, years of education, and memory functions. In addition, as shown in our additional sensitivity analyses (Supplementary Results-2 and Fig. S2, see online Supplementary Material ), the rich-club connectivity of the precuneus is associated with the severity of depressive symptoms but not the degree of SMCs. Taken together, our findings suggest that alterations in the structural rich-club connections of the precuneus are associated with the manifestation of depressive symptoms in SMCs. Our results are in alignment with the previous study, which demonstrated that connectome structures related to the default mode network (DMN), including the precuneus were significantly reduced in MDD patients compared with healthy controls (Korgaonkar et al., 2014). Furthermore, our results support previous findings, which identified several nodes—including the precuneus—that exhibited significant differences between MDD patients and healthy controls (GadElkarim et al., 2012), and those that found associations between depressive symptoms in AD patients with cortical thinning in the temporal and parietal regions, including the precuenus (Lebedeva et al., 2014). Therefore, our findings imply that depressive symptoms in middle-aged individuals with SMCs are more significant in meaning than simply being one of the reflective features related to SMCs and represent structural alterations in connectivity strength of the precuneus in rich-club organization.
In addition, it is noteworthy that the precuneus is one of the vulnerable regions for amyloid deposition even in healthy elderly individuals (Sperling et al., 2009). A previous study has shown that amyloid deposition in the precuneus is well correlated with structural alterations in the brain, such as cortical atrophy in the precuneus among individuals who are in the preclinical stages of AD (Chetelat et al., 2010). Furthermore, disruptions of white matter connectivity in the DMN, including the precuneus, have been reported in the nondemented elderly with APOE ε4 carrier (Brown et al., 2011), which is a well-known genetic risk factor for AD as can be found from our findings of lower precuneus connectivity strength in the SMCD group. Previous studies may further support this finding, as it has been reported that increased amyloid deposition in the precuneus is present in patients with a lifetime history of major depression compared with healthy controls (Wu et al., 2014).
Similar to our results from the rich-club analysis, we expected that depressive symptoms in SMCs may influence objective measures of cognitive performance. However, although the SMCD group showed significantly lower scores only on the SRM task among the individual tasks that comprised the composite scores, there were no statistically significant differences in performance on visual and working memory composites between the SMCO and SMCD groups. As aforementioned, the current study only performed visual and working memory tasks to determine cognitive function, with the exclusion of other cognitive domains that have been previously investigated in the past such as processing speed and verbal memory (McDermott & Ebmeier, 2009; Nebes et al., 2000). As such, there may be differences in performance in other cognitive domains between the SMCD and SMCO groups that the current study may have missed, such as between-group differences in verbal memory or processing speed. In addition, it is noteworthy that the current sample size may be relatively small not be large enough to detect such a small effect size of differences in visual and working memory performance between the groups. The future studies with a larger sample size will be needed to determine the cognitive differences in individuals with SMCs according to the presence of concomitant depressive symptoms.
One of key findings of this study is that the SMCD group showed a positive correlation between the connectivity strength of the precuneus and working memory performance. This finding may support the previous study which showed a correlation between decreased white matter integrity connecting the DMN (including the precuneus) and cingulo-opercular network and lower working memory performance in individuals with late onset depression (Yin et al., 2016). In addition, another previous functional neuroimaging study also revealed that depressive symptoms and verbal memory impairment in non-demented elderly had interactive effects on the DMN (Goveas et al., 2011). Given that the precuneus is known to be a core region of DMN (Utevsky, Smith, & Huettel, 2014) and alterations in rich-club organization could occur before the occurrence of any pronounced symptoms related to a disease (McColgan et al., 2015), our findings may imply that depressive symptoms could render individuals with SMCs more vulnerable to working memory impairment by altering the structural connectivity strength of the precuneus in rich-club organizations. However, it is noteworthy that the current study design is a cross-sectional study, causal-relationship between the structural connectivity of the precuneus, depressive symptoms and working memory impairment could not be investigated in this study. Further refined longitudinal studies would be helpful to elucidate the detailed neurobiological mechanisms of working memory impairment in individuals with SMCs and concomitant depressive symptoms.
Although the hippocampus was not categorized as part of the rich-club nodes in the current study, we analyzed the connectivity strength of the hippocampus between the SMCD and SMCO group to provide more insight with regard to the role of the hippocampus in SMCs and depressive symptoms. We expected that the SMCD group would show lower connectivity strength of the hippocampus as compared to the SMCO group, considering that numerous previous studies have indicated that the hippocampus is one of the key brain regions in SMCs and depression (Sawyer et al., 2012; Stewart et al., 2011; von Gunten et al., 2000; von Gunten & Ron, 2004). However, contrary to our expectation, there were no differences in connectivity strength of the hippocampus between the SMCO and SMCD group. This finding may be partly consistent with other previous studies, which suggested that the hippocampus may be affected as depression progresses rather than in the subclinical stage of depression (Hayakawa et al., 2013; Taki et al., 2005). In addition, previous studies have reported that asymptomatic APOE ε4 carriers in healthy elderly showed structural changes in the precuneus exclusively, and not in the hippocampus (Brown et al., 2011; Chen et al., 2017). The current study may support that the alteration in connectivity strength of the hippocampus is progression-specific in the case of SMCs and depression.
This study has some limitations. First, the current study design did not recruit subjects with depressive symptoms but without any complaints of memory impairment as well as healthy individuals. As such, we could not investigate the individual effects of SMCs and depressive symptoms on the structural brain networks. Future studies with balanced samples that include the four individual groups (SMCs only vs. depressive symptoms only vs. SMCs with depressive symptoms vs. controls) would be practical in examining the exact additive or interactive effects of SMCs and depressive symptoms on the structural brain network. Furthermore, although the test scores of both SMCO and SMCD groups in the current study were lower than those of healthy individuals with a similar age range who have undergone the tasks in other studies (Rahman et al., 1999; Saunders & Summers, 2010; Swainson et al., 2001) (Table S1, see online Supplementary Material ), it should be noted that the precise differences in cognitive functions of the SMCs groups from healthy individuals could not be determined in the current study. In addition, as aforementioned, the current study only examined visual and working memory composites to measure cognitive domains. Therefore, the possibility that the SMCD group might have lower performances in other cognitive domains, such as verbal memory or processing speed, compared to those in the SMCO group, have not been considered. As such, the examination of other cognitive domains is warranted in future studies. Fourth, as mentioned, a causal relationship between depressive symptoms and alterations in the brain network of individuals with SMCs could not be examined due to the cross-sectional nature of the study design. Our findings can only suggest that alterations in the structural rich-club connections of the precuneus are associated with the manifestation of depressive symptoms in SMCs. Fifth, information related to AD pathology, such as beta-amyloid in cerebrospinal fluid or genetic information, such as APOE was not collected, despite its potential role in affecting the rich-club organization as well as cognitive impairment (Chen et al., 2015). Sixth, although our results suggest that individuals with SMCD may be vulnerable to alterations in rich-club connections in the precuneus, the connectivity strength of rich-club connections in the precuneus was not significantly correlated with depressive symptoms. This lack of correlation between depressive symptoms and the precuneus connectivity may suggest that alterations in the structural circuits based on the binary diagnosis of depressive symptoms may not be the results of the alterations of the brain in a relation to the severity of depressive symptoms. This is consistent with previous research that investigated the structural network in MDD patients, which showed that network connections did not correlate with MDD severity nor time since the onset of MDD (Korgaonkar et al., 2014). It also is noteworthy that this lack of correlation may be alternatively explained by the scores of depressive symptoms in the SMCD group, which were skewed to the left side. Seventh, individuals with SMCs in the current study were enrolled under the criterion of having a score of one or higher on the SMCQ. Although previous studies have often used only one question in the enrollment of individuals with SMCs (Geerlings et al., 1999; Laske et al., 2015; Schultz et al., 2015), it should be noted that using a cutoff score of 1 or higher in a total of 14 scores from the SMCQ questionnaire may be insufficient in classifying SMCs from healthy control. However, the SMCQ scores of the current participants are similar in range to other previous studies based on individuals with SMC with normal cognitive function (Table S2, see online Supplementary Material ) (Kang et al., 2017; Seo et al., 2017; Yim et al., 2017). Finally, because the effect size of the between-group differences in the precuneus connectivity is relatively small (ηp2=0.03) (Cohen, 1973; Levine & Hullett, 2002), it should be noted that the current study has only 51.6% of statistical power to detect this difference. Therefore, replication studies with a larger sample size using a more conservative method to correct for multiple comparisons, such as a Bonferroni-class approach, are warranted to obtain a more robust finding.
Nonetheless, the current study has the strength that it investigated the neural correlates of depressive symptoms in middle-aged individuals with SMCs, a population and approach that had not been well elucidated before. Our study suggests that reduced connectivity strength of the precuneus in rich-club connections, an area that is essential for integrating information for cognitive and emotional processes (Cavanna & Trimble, 2006; van der Velde et al., 2013), is observed in individuals with SMCs and depressive symptoms, relative to those in SMCs without depressive symptoms. Given that the precuneus is one of the commonly affected brain regions during the early stages of AD (Sperling et al., 2009) and increased amyloid deposition in the precuneus was reported in patients with MDD (Wu et al., 2014), our findings may suggest that the concomitant depressive symptoms in middle-aged individuals with SMCs may reflect early structural network changes in rich-club organization related to AD. Considering that SMCs can arise as early as in middle-age and are associated with the future onset of cognitive impairment that occur as late as two decades subsequent to the SMCs (Kaup et al., 2015), earlier diagnosis as well as appropriate management of its risk factors such as depressive symptoms could be important in mitigating further cognitive impairment (Byers & Yaffe, 2011; Hughes & Ganguli, 2009). In this respect, our findings may provide important evidence of alterations in the structural brain network related to depressive symptoms in middle-aged individuals with SMCs, which may further contribute to understanding the neural mechanisms of concomitant depressive symptoms in SMCs.
References
Balash, Y., Mordechovich, M., Shabtai, H., Giladi, N., Gurevich, T., & Korczyn, A. D. (2013). Subjective memory complaints in elders: depression, anxiety, or cognitive decline? Acta Neurol Scand, 127(5), 344-350. https://doi.org/10.1111/ane.12038
Balash, Y., Mordechovich, M., Shabtai, H., Merims, D., & Giladi, N. (2010). Subjective memory decline in healthy community-dwelling elders. What does this complain mean? Acta Neurologica Scandinavica, 121(3), 194-197.
Batalle, D., Eixarch, E., Figueras, F., Munoz-Moreno, E., Bargallo, N., Illa, M., . . . Gratacos, E. (2012). Altered small-world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. NeuroImage, 60(2), 1352-1366. https://doi.org/10.1016/j.neuroimage.2012.01.059
Brigola, A. G., Manzini, C. S. S., Oliveira, G. B. S., Ottaviani, A. C., Sako, M. P., & Vale, F. A. C. (2015). Subjective memory complaints associated with depression and cognitive impairment in the elderly: A systematic review. Dement Neuropsychol, 9(1), 51-57. https://doi.org/10.1590/S1980-57642015DN91000009
Brown, J. A., Terashima, K. H., Burggren, A. C., Ercoli, L. M., Miller, K. J., Small, G. W., & Bookheimer, S. Y. (2011). Brain network local interconnectivity loss in aging APOE-4 allele carriers. Proceedings of the National Academy of Sciences, 108(51), 20760-20765.
Buckley, R., Saling, M. M., Ames, D., Rowe, C. C., Lautenschlager, N. T., Macaulay, S. L., . . . Ellis, K. A. (2013). Factors affecting subjective memory complaints in the AIBL aging study: biomarkers, memory, affect, and age. Int Psychogeriatr, 25(8), 1307-1315. https://doi.org/10.1017/s1041610213000665
Buckley, R. F., Maruff, P., Ames, D., Bourgeat, P., Martins, R. N., Masters, C. L., . . . Ellis, K. A. (2016). Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer's disease. Alzheimers Dement. https://doi.org/10.1016/j.jalz.2015.12.013
Byers, A. L., & Yaffe, K. (2011). Depression and risk of developing dementia. Nature Reviews Neurology, 7(6), 323.
Cavanna, A. E., & Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564-583. https://doi.org/10.1093/brain/awl004
Chen, Y., Chen, K., Zhang, J., Li, X., Shu, N., Wang, J., . . . Reiman, E. M. (2015). Disrupted functional and structural networks in cognitively normal elderly subjects with the APOE ε4 allele. Neuropsychopharmacology, 40(5), 1181-1191. https://doi.org/10.1038/npp.2014.302
Chen, Y., Liu, Z., Zhang, J., Chen, K., Yao, L., Li, X., . . . Zhang, Z. (2017). Precuneus degeneration in nondemented elderly individuals with APOE ε4: Evidence from structural and functional MRI analyses. Hum Brain Mapp, 38(1), 271-282. https://doi.org/10.1002/hbm.23359
Chetelat, G., Villemagne, V. L., Bourgeat, P., Pike, K. E., Jones, G., Ames, D., . . . Rowe, C. C. (2010). Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol, 67(3), 317-324. https://doi.org/10.1002/ana.21955
Chin, J., Oh, K. J., Seo, S. W., & Na, D. L. (2014). Are depressive symptomatology and self-focused attention associated with subjective memory impairment in older adults? Int Psychogeriatr, 26(4), 573-580. https://doi.org/10.1017/S104161021300241X
Choi, S. H., Na, D. L., Lee, B. H., Hahm, D. S., Jeong, J. H., Yoon, S. J., . . . Group, D. R. (2001). Estimating the validity of the Korean version of expanded clinical dementia rating (CDR) scale. Journal of the Korean Neurological Association, 19(6), 585-591.
Christopher, G., & MacDonald, J. (2005). The impact of clinical depression on working memory. Cogn Neuropsychiatry, 10(5), 379-399. https://doi.org/10.1080/13546800444000128
Cohen, J. (1973). Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educational and psychological measurement.
Cook, S., & Marsiske, M. (2006). Subjective memory beliefs and cognitive performance in normal and mildly impaired older adults. Aging Ment Health, 10(4), 413-423. https://doi.org/10.1080/13607860600638487
Crossley, N. A., Mechelli, A., Scott, J., Carletti, F., Fox, P. T., McGuire, P., & Bullmore, E. T. (2014). The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain, 137(Pt 8), 2382-2395. https://doi.org/10.1093/brain/awu132
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., . . . Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968-980. https://doi.org/10.1016/j.neuroimage.2006.01.021
Dotson, V. M., Davatzikos, C., Kraut, M. A., & Resnick, S. M. (2009). Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J Psychiatry Neurosci, 34(5), 367-375.
Fischer, F. U., Wolf, D., Scheurich, A., Fellgiebel, A., & Alzheimer's Disease Neuroimaging Initiative, A. (2015). Altered whole-brain white matter networks in preclinical Alzheimer's disease. NeuroImage: clinical, 8, 660-666.
GadElkarim, J. J., Schonfeld, D., Ajilore, O., Zhan, L., Zhang, A. F., Feusner, J. D., . . . Leow, A. D. (2012). A framework for quantifying node-level community structure group differences in brain connectivity networks. Med Image Comput Comput Assist Interv, 15(Pt 2), 196-203.
Geerlings, M. I., Jonker, C., Bouter, L. M., Adèr, H. J., & Schmand, B. (1999). Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. American Journal of Psychiatry, 156(4), 531-537.
Gong, G., He, Y., Concha, L., Lebel, C., Gross, D. W., Evans, A. C., & Beaulieu, C. (2009). Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex, 19(3), 524-536. https://doi.org/10.1093/cercor/bhn102
Goveas, J., Xie, C., Wu, Z., Douglas Ward, B., Li, W., Franczak, M. B., . . . Li, S. J. (2011). Neural correlates of the interactive relationship between memory deficits and depressive symptoms in nondemented elderly: resting fMRI study. Behav Brain Res, 219(2), 205-212. https://doi.org/10.1016/j.bbr.2011.01.008
Hafkemeijer, A., Altmann-Schneider, I., Oleksik, A. M., van de Wiel, L., Middelkoop, H. A., van Buchem, M. A., . . . Rombouts, S. A. (2013). Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect, 3(4), 353-362. https://doi.org/10.1089/brain.2013.0144
Hagmann, P., Cammoun, L., Gigandet, X., Meuli, R., Honey, C. J., Wedeen, V. J., & Sporns, O. (2008). Mapping the structural core of human cerebral cortex. PLoS Biol, 6(7), e159. https://doi.org/10.1371/journal.pbio.0060159
Hagmann, P., Kurant, M., Gigandet, X., Thiran, P., Wedeen, V. J., Meuli, R., & Thiran, J. P. (2007). Mapping human whole-brain structural networks with diffusion MRI. PLoS One, 2(7), e597. https://doi.org/10.1371/journal.pone.0000597
Hahn, O. S., Ahn, J. H., Song, S. H., Cho, M. J., Kim, J. K., Bae, J. N., . . . Hahm, B. J. (2000). Development of Korean version of structured clinical interview schedule for DSM-IV axis I disorder: interrater reliability. Journal of Korean Neuropsychiatric Association, 39(2), 362-372.
Hammar, A., Isaksen, L., Schmid, M., Ardal, G., & Strand, M. (2011). Patients with major depression show intact memory performance--given optimal conditions. Appl Neuropsychol, 18(3), 191-196. https://doi.org/10.1080/09084282.2011.595445
Hayakawa, Y. K., Sasaki, H., Takao, H., Mori, H., Hayashi, N., Kunimatsu, A., . . . Ohtomo, K. (2013). Structural brain abnormalities in women with subclinical depression, as revealed by voxel-based morphometry and diffusion tensor imaging. Journal of affective disorders, 144(3), 263-268.
Hohman, T. J., Beason-Held, L. L., & Resnick, S. M. (2011). Cognitive complaints, depressive symptoms, and cognitive impairment: are they related? Journal of the American Geriatrics Society, 59(10), 1908-1912.
Houenou, J., Wessa, M., Douaud, G., Leboyer, M., Chanraud, S., Perrin, M., . . . Paillere-Martinot, M. L. (2007). Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry, 12(11), 1001-1010. https://doi.org/10.1038/sj.mp.4002010
Hughes, T. F., & Ganguli, M. (2009). Modifiable Midlife Risk Factors for Late-Life Cognitive Impairment and Dementia. Curr Psychiatry Rev, 5(2), 73-92.
Hurtz, S., Woo, E., Kebets, V., Green, A. E., Zoumalan, C., Wang, B., . . . Apostolova, L. G. (2014). Age effects on cortical thickness in cognitively normal elderly individuals. Dement Geriatr Cogn Dis Extra, 4(2), 221-227. https://doi.org/10.1159/000362872
Jessen, F., Wiese, B., Bachmann, C., Eifflaender-Gorfer, S., Haller, F., Kolsch, H., . . . Dementia in Primary Care Patients Study, G (2010). Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry, 67(4), 414-422. https://doi.org/10.1001/archgenpsychiatry.2010.30
Jessen, F., Wiese, B., Cvetanovska, G., Fuchs, A., Kaduszkiewicz, H., Kolsch, H., . . . Bickel, H. (2007). Patterns of subjective memory impairment in the elderly: association with memory performance. Psychol Med, 37(12), 1753-1762. https://doi.org/10.1017/S0033291707001122
Juncos-Rabadan, O., Pereiro, A. X., Facal, D., Rodriguez, N., Lojo, C., Caamano, J. A., . . . Eiroa, P. (2012). Prevalence and correlates of cognitive impairment in adults with subjective memory complaints in primary care centres. Dement Geriatr Cogn Disord, 33(4), 226-232. https://doi.org/10.1159/000338607
Kalska, H., Punamaki, R.-L., Makinen-Pelli, T., & Saarinen, M. (1999). Memory and metamemory functioning among depressed patients. Applied Neuropsychology, 6(2), 96-107.
Kang, S.-H., Yoon, I.-Y., Lee, S. D., Kim, T., Lee, C. S., Han, J. W., . . . Kim, C.-H. (2017). Subjective memory complaints in an elderly population with poor sleep quality. Aging Ment Health, 21(5), 532-536.
Kaup, A. R., Nettiksimmons, J., LeBlanc, E. S., & Yaffe, K. (2015). Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology, 85(21), 1852-1858.
Kim, C., Lee, J. Y., Ha, T. H., Choi, J. H., Yu, E.-M., Lee, S. J., . . . Park, M. Y. (2009). The usefulness of the Cambridge Neuropsychological Test Automated Battery (CANTAB) for assessing cognitive functions in the elderly: A pilot study. Journal of the Korean Geriatrics Society, 13(2), 69-78.
Kim, E. Y., & Cho, E. H. (2012). Test/re-test Reliability of the CANTAB Batteries to Evaluate the Neuropsychological Function for Korean Adult.The Korean Journal of Measurement and Evaluation in Physical Education and Sport Science, 14(1), 11-17.
Kim, H. S., An, Y. M., Kwon, J. S., & Shin, M.-S. (2014). A preliminary validity study of the cambridge neuropsychological test automated battery for the assessment of executive function in schizophrenia and bipolar disorder. Psychiatry Investigation, 11(4), 394-401.
Korgaonkar, M. S., Fornito, A., Williams, L. M., & Grieve, S. M. (2014). Abnormal Structural Networks Characterize Major Depressive Disorder: A Connectome Analysis. Biol Psychiatry, 76(7), 567-574. https://doi.org/10.1016/j.biopsych.2014.02.018
Kurt, P., Yener, G., & Oguz, M. (2011). Impaired digit span can predict further cognitive decline in older people with subjective memory complaint: a preliminary result. Aging Ment Health, 15(3), 364-369. https://doi.org/10.1080/13607863.2010.536133
Laske, C., Sohrabi, H. R., Jasielec, M. S., Muller, S., Koehler, N. K., Graber, S., . . . Dominantly Inherited Alzheimer Network, D. (2015). Diagnostic Value of Subjective Memory Complaints Assessed with a Single Item in Dominantly Inherited Alzheimer's Disease: Results of the DIAN Study. Biomed Res Int, 2015, 828120. https://doi.org/10.1155/2015/828120
Latora, V., & Marchiori, M. (2001). Efficient behavior of small-world networks. Phys Rev Lett, 87(19), 198701.
Lavretsky, H., & Kumar, A. (2002). Clinically significant non-major depression: old concepts, new insights. Am J Geriatr Psychiatry, 10(3), 239-255.
Lavretsky, H., Siddarth, P., Kepe, V., Ercoli, L. M., Miller, K. J., Burggren, A. C., . . . Small, G. W. (2009). Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. Am J Geriatr Psychiatry, 17(6), 493-502.
Lebedeva, A., Westman, E., Lebedev, A. V., Li, X., Winblad, B., Simmons, A., . . . Aarsland, D. (2014). Structural brain changes associated with depressive symptoms in the elderly with Alzheimer's disease. J Neurol Neurosurg Psychiatry, 85(8), 930-935. https://doi.org/10.1136/jnnp-2013-307110
Lehrner, J., Moser, D., Klug, S., Gleiß, A., Auff, E., Dal-Bianco, P., & Pusswald, G. (2014). Subjective memory complaints, depressive symptoms and cognition in patients attending a memory outpatient clinic. International Psychogeriatrics, 26(3), 463-473.
Levine, T. R., & Hullett, C. R. (2002). Eta squared, partial eta squared, and misreporting of effect size in communication research. Human Communication Research, 28(4), 612-625.
Lista, S., Molinuevo, J. L., Cavedo, E., Rami, L., Amouyel, P., Teipel, S. J., . . . Hampel, H. (2015). Evolving Evidence for the Value of Neuroimaging Methods and Biological Markers in Subjects Categorized with Subjective Cognitive Decline. J Alzheimers Dis, 48 Suppl 1, S171-191. https://doi.org/10.3233/jad-150202
Lo, C. Y., Wang, P. N., Chou, K. H., Wang, J., He, Y., & Lin, C. P. (2010). Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer's disease. J Neurosci, 30(50), 16876-16885. https://doi.org/10.1523/JNEUROSCI.4136-10.2010
McColgan, P., Seunarine, K. K., Razi, A., Cole, J. H., Gregory, S., Durr, A., . . . Tabrizi, S. J. (2015). Selective vulnerability of Rich Club brain regions is an organizational principle of structural connectivity loss in Huntington's disease. Brain, 138(Pt 11), 3327-3344. https://doi.org/10.1093/brain/awv259
McDermott, L. M., & Ebmeier, K. P. (2009). A meta-analysis of depression severity and cognitive function. J Affect Disord, 119(1-3), 1-8. https://doi.org/10.1016/j.jad.2009.04.022
Montejo Carrasco, P., Montenegro-Pena, M., Lopez-Higes, R., Estrada, E., Prada Crespo, D., Montejo Rubio, C., & Garcia Azorin, D. (2017). Subjective Memory Complaints in healthy older adults: Fewer complaints associated with depression and perceived health, more complaints also associated with lower memory performance. Arch Gerontol Geriatr, 70, 28-37. https://doi.org/10.1016/j.archger.2016.12.007
Montejo, P., Montenegro, M., Fernández-Blázquez, M. A., Turrero-Nogués, A., Yubero, R., Huertas, E., & Maestú, F. (2014). Association of perceived health and depression with older adults’ subjective memory complaints: contrasting a specific questionnaire with general complaints questions. European Journal of Ageing, 11(1), 77-87. https://doi.org/10.1007/s10433-013-0286-4
Mori, S., Crain, B. J., Chacko, V. P., & van Zijl, P. C. (1999). Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol, 45(2), 265-269.
Morris, J. C. (1997). Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr, 9 Suppl 1, 173-176; discussion 177-178.
Nebes, R. D., Butters, M. A., Mulsant, B. H., Pollock, B. G., Zmuda, M. D., Houck, P. R., & Reynolds, C. F., 3rd. (2000). Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med, 30(3), 679-691.
Nichols, T. E., & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp, 15(1), 1-25.
Onnela, J. P., Saramaki, J., Kertesz, J., & Kaski, K. (2005). Intensity and coherence of motifs in weighted complex networks. Phys Rev E Stat Nonlin Soft Matter Phys, 71(6 Pt 2), 065103. https://doi.org/10.1103/PhysRevE.71.065103
Owen, A. M., Sahakian, B. J., Semple, J., Polkey, C. E., & Robbins, T. W. (1995). Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia, 33(1), 1-24.
Park, J. (1989). Standardization of Korean version of the mini-mental state examination (MMSE-K) for use in the elderly. Part II. Diagnostic validity. Korean J Neuropsych Assoc, 28, 125-135.
Porter, R. J., Gallagher, P., Thompson, J. M., & Young, A. H. (2003). Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry, 182(3), 214-220.
Rahman, S., Sahakian, B. J., Hodges, J. R., Rogers, R. D., & Robbins, T. W. (1999). Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain, 122 ( Pt 8), 1469-1493.
Reisberg, B., & Gauthier, S. (2008). Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer's disease. Int Psychogeriatr, 20(1), 1-16. https://doi.org/10.1017/S1041610207006412
Robbins, T. W., James, M., Owen, A. M., Sahakian, B. J., McInnes, L., & Rabbitt, P. (1994). Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia, 5(5), 266-281.
Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage, 52(3), 1059-1069. https://doi.org/10.1016/j.neuroimage.2009.10.003
Saunders, N. L., & Summers, M. J. (2010). Attention and working memory deficits in mild cognitive impairment. J Clin Exp Neuropsychol, 32(4), 350-357. https://doi.org/10.1080/13803390903042379
Sawyer, K., Corsentino, E., Sachs-Ericsson, N., & Steffens, D. C. (2012). Depression, Hippocampal Volume Changes, and Cognitive Decline in a Clinical Sample of Older Depressed Outpatients and Non-depressed Controls. Aging Ment Health, 16(6), 753-762. https://doi.org/10.1080/13607863.2012.678478
Scheef, L., Spottke, A., Daerr, M., Joe, A., Striepens, N., Kolsch, H., . . . Jessen, F. (2012). Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology, 79(13), 1332-1339. https://doi.org/10.1212/WNL.0b013e31826c1a8d
Schultz, S. A., Oh, J. M., Koscik, R. L., Dowling, N. M., Gallagher, C. L., Carlsson, C. M., . . . Okonkwo, O. C. (2015). Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-aged adults at risk for AD. Alzheimers Dement (Amst), 1(1), 33-40. https://doi.org/10.1016/j.dadm.2014.11.010
Seo, E. H., Kim, H., Choi, K. Y., Lee, K. H., & Choo, I. H. (2017). Association of subjective memory complaint and depressive symptoms with objective cognitive functions in prodromal Alzheimer's disease including pre-mild cognitive impairment. J Affect Disord, 217, 24-28. https://doi.org/10.1016/j.jad.2017.03.062
Sharma, A. (2013). Cambridge Neuropsychological Test Automated Battery. In F. R. Volkmar (Ed.), Encyclopedia of autism spectrum disorders (pp. 498-515). New York, NY: Springer New York.
Shu, N., Liu, Y., Li, K., Duan, Y., Wang, J., Yu, C., . . . He, Y. (2011). Diffusion tensor tractography reveals disrupted topological efficiency in white matter structural networks in multiple sclerosis. Cereb Cortex, 21(11), 2565-2577. doi:bhr039 [pii]10.1093/cercor/bhr039
Sousa, M., Pereira, A., & Costa, R. (2015). Subjective Memory Complaint and Depressive Symptoms among Older Adults in Portugal. Curr Gerontol Geriatr Res, 2015, 296581. https://doi.org/10.1155/2015/296581
Sperling, R. A., LaViolette, P. S., O'Keefe, K., O'Brien, J., Rentz, D. M., Pihlajamaki, M., . . . Hedden, T. (2009). Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron, 63(2), 178-188.
Stewart, R., Dufouil, C., Godin, O., Ritchie, K., Maillard, P., Delcroix, N., . . . Tzourio, C. (2008). Neuroimaging correlates of subjective memory deficits in a community population. Neurology, 70(18), 1601-1607. https://doi.org/10.1212/01.wnl.0000310982.99438.54
Stewart, R., Godin, O., Crivello, F., Maillard, P., Mazoyer, B., Tzourio, C., & Dufouil, C. (2011). Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br J Psychiatry, 198(3), 199-205. https://doi.org/10.1192/bjp.bp.110.078683
Sun, Y., Dai, Z., Li, Y., Sheng, C., Li, H., Wang, X., . . . Han, Y. (2016). Subjective cognitive decline: mapping functional and structural brain changes-a combined resting-state functional and structural MR imaging study. Radiology, 151771. https://doi.org/10.1148/radiol.2016151771
Sung, H. M., Kim, J., Park, Y., Bai, D., Lee, S., & Ahn, H. (2008). A Study on the Reliability and the Validity of Korean Version of the Beck Depression Inventory-II(BDI -II). Journal of the Korean sociecty of biological therapies in psychiatry, 14(2), 201-212.
Swainson, R., Hodges, J. R., Galton, C. J., Semple, J., Michael, A., Dunn, B. D., . . . Sahakian, B. J. (2001). Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord, 12(4), 265-280. https://doi.org/10.1159/000051269
Szymkowicz, S. M., Woods, A. J., Dotson, V. M., Porges, E. C., Nissim, N. R., O'Shea, A., . . . Ebner, N. C. (2018). Associations between subclinical depressive symptoms and reduced brain volume in middle-aged to older adults. Aging Ment Health, 1-12. https://doi.org/10.1080/13607863.2018.1432030
Taki, Y., Kinomura, S., Awata, S., Inoue, K., Sato, K., Ito, H., . . . Arai, H. (2005). Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. Journal of affective disorders, 88(3), 313-320.
Utevsky, A. V., Smith, D. V., & Huettel, S. A. (2014). precuneus is a functional core of the default-mode network. The Journal of Neuroscience, 34(3), 932-940. https://doi.org/10.1523/JNEUROSCI.4227-13.2014
van den Heuvel, M. P., & Sporns, O. (2011). Rich-club organization of the human connectome. J Neurosci, 31(44), 15775-15786. https://doi.org/10.1523/jneurosci.3539-11.2011
van den Heuvel, M. P., Sporns, O., Collin, G., Scheewe, T., Mandl, R. C., Cahn, W., . . . Kahn, R. S. (2013). Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry, 70(8), 783-792. https://doi.org/10.1001/jamapsychiatry.2013.1328
van der Velde, J., Servaas, M. N., Goerlich, K. S., Bruggeman, R., Horton, P., Costafreda, S. G., & Aleman, A. (2013). Neural correlates of alexithymia: A meta-analysis of emotion processing studies. Neuroscience & Biobehavioral Reviews, 37(8), 1774-1785. https://doi.org/10.1016/j.neubiorev.2013.07.008
von Gunten, A., Fox, N. C., Cipolotti, L., & Ron, M. A. (2000). A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J Neuropsychiatry Clin Neurosci, 12(4), 493-498. https://doi.org/10.1176/jnp.12.4.493
von Gunten, A., & Ron, M. A. (2004). Hippocampal volume and subjective memory impairment in depressed patients. Eur Psychiatry, 19(7), 438-440. https://doi.org/10.1016/j.eurpsy.2004.05.003
Waldorff, F. B., Siersma, V., Vogel, A., & Waldemar, G. (2012). Subjective memory complaints in general practice predicts future dementia: a 4-year follow-up study. Int J Geriatr Psychiatry, 27(11), 1180-1188. https://doi.org/10.1002/gps.3765
Wang, R., Beener, T., Sorensen, A. G., & Wedeen, V. J. (2007). Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med, 15, 3720.
Watts, D. J., & Strogatz, S. H. (1998). Collective dynamics of 'small-world' networks. Nature, 393(6684), 440-442. https://doi.org/10.1038/30918
Westfall, P. H., Young, S. S., & Wright, S. P. (1993). On adjusting P-values for multiplicity. Biometrics, 49(3), 941-945.
Wu, K.-Y., Hsiao, T., Chen, C.-S., Chen, C.-H., Hsieh, C.-J., Wai, Y.-Y., . . . Liu, C.-Y. (2014). Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18 F-florbetapir (AV-45/Amyvid) positron emission tomography. European journal of nuclear medicine and molecular imaging, 41(4), 714-722.
Yan, C., Gong, G., Wang, J., Wang, D., Liu, D., Zhu, C., . . . He, Y. (2011). Sex- and brain size-related small-world structural cortical networks in young adults: a DTI tractography study. Cereb Cortex, 21(2), 449-458. https://doi.org/10.1093/cercor/bhq111
Yasuno, F., Kazui, H., Yamamoto, A., Morita, N., Kajimoto, K., Ihara, M., . . . Kishimoto, T. (2015). Resting-state synchrony between the retrosplenial cortex and anterior medial cortical structures relates to memory complaints in subjective cognitive impairment. Neurobiol Aging, 36(6), 2145-2152. https://doi.org/10.1016/j.neurobiolaging.2015.03.006
Yim, S. J., Yi, D., Byun, M. S., Choe, Y. M., Choi, H. J., Baek, H., . . . Lee, D. Y. (2017). Screening ability of subjective memory complaints, informant-reports for cognitive decline, and their combination in memory clinic setting. Psychiatry Investigation, 14(5), 640-646.
Yin, Y., He, X., Xu, M., Hou, Z., Song, X., Sui, Y., . . . Zhang, Y. (2016). Structural and functional connectivity of default mode network underlying the cognitive impairment in late-onset depression. Scientific reports, 6, 37617.
Yoon, S., Kim, J. E., Hwang, J., Kim, T. S., Kang, H. J., Namgung, E., . . . Lyoo, I. K. (2016). Effects of creatine monohydrate augmentation on brain metabolic and network outcome measures in women with major depressive disorder. Biol Psychiatry, 80(6), 439-447. https://doi.org/10.1016/j.biopsych.2015.11.027
Youn, J. C., Kim, K. W., Lee, D. Y., Jhoo, J. H., Lee, S. B., Park, J. H., . . . Woo, J. I. (2009). Development of the Subjective Memory Complaints Questionnaire. Dement Geriatr Cogn Disord, 27(4), 310-317. https://doi.org/10.1159/000205512
Zhang, Z., Liao, W., Chen, H., Mantini, D., Ding, J. R., Xu, Q., . . . Lu, G. (2011). Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain, 134(Pt 10), 2912-2928. https://doi.org/10.1093/brain/awr223
Zhou, H., Li, R., Ma, Z., Rossi, S., Zhu, X., & Li, J. (2016). Smaller gray matter volume of hippocampus/parahippocampus in elderly people with subthreshold depression: a cross-sectional study. BMC Psychiatry, 16(1), 219.
Zlatar, Z. Z., Moore, R. C., Palmer, B. W., Thompson, W. K., & Jeste, D. V. (2014). Cognitive complaints correlate with depression rather than concurrent objective cognitive impairment in the successful aging evaluation baseline sample. J Geriatr Psychiatry Neurol, 27(3), 181-187. https://doi.org/10.1177/0891988714524628
Zlatar, Z. Z., Muniz, M., Galasko, D., & Salmon, D. P. (2017). Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. J Gerontol B Psychol Sci Soc Sci. https://doi.org/10.1093/geronb/gbw207
Funding
This work was supported from the ICT R&D program of Institute for Information & communications Technology Promotion (B0132-15-1001 to SY) and through the the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2015M3C7A1028376 to JYK) and by the Ministry of Education (2015R1D1A1A01061198 to GHK, and 2016R1A2B2012575 to SY).
Author information
Authors and Affiliations
Contributions
GHK and JYK: Conception and design, data analysis and interpretation, and manuscript writing; JEK, JM, BK, JI, HJ, EN, SL, IK and IL: Collection and assembly of data and data analysis and interpretation; JH and SY: Conception and design, administrative support, manuscript writing, and final approval of manuscript
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 60 kb)
Rights and permissions
About this article
Cite this article
Kim, G.H., Kim, J.Y., Kim, J.E. et al. Alterations in structural rich-club connectivity of the precuneus are associated with depressive symptoms among individuals with subjective memory complaints. Cogn Affect Behav Neurosci 19, 73–87 (2019). https://doi.org/10.3758/s13415-018-0645-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-018-0645-x