Abstract
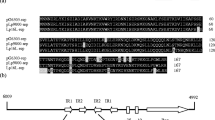
Complete nucleotide sequence of plasmid pGP2 from Acetobacter estunensis GP2 was identified after initial cloning of EcoRI fragment followed by preparation of deletion derivatives. Its size was defined to 2,797 bp and several sites for several restriction enzymes were revealed by DNA sequencing. Sequence analysis predicts three putative open reading frames (ORFs). ORF1 shows significant identity with the bacterial excinuclease α-subunit, ORF2 is a putative replication protein with low similarity with other Acetobacter plasmid’s replication proteins, and ORF3 encodes a class B acid phosphatase/phosphotransferase. The replication module comprises a DnaA box like sequence, direct repeats, a potential prokaryotic promoter and a rep gene. The rep module is similar with several θ-replicating, iteron-containing modules from plasmids, suggesting pGP2 replication may follow the same course. Any phenotypic character determinant gene is absent in pGP2, suggesting this plasmid to be cryptic. However, a pGP2 derivative plasmid, containing the putative pGP2 rep region, can replicate and is stably maintained in Acetobacter and Escherichia coli strains; it can also carry foreign DNA fragments. Thus, pGP2-X could serve as a cloning shuttle vector between these bacteria. Prepared deletion derivatives of plasmid pGP2 suggested that Rep protein is essential for plasmid replication in host bacteria. In its natural host, A. estunensis GP2, pGP2 maintains a four-times lower copy number than in E. coli.
Similar content being viewed by others
Abbreviations
- AAB:
-
acetic acid bacteria
- CCM:
-
Czech Collection of Microorganisms
- HTH:
-
helix-turn-helix
- ori :
-
origin of replication
- KnR :
-
kanamycin resistance
- ORF:
-
open reading frame
- RBS:
-
ribosome-binding site
- rep :
-
replication
References
Abeles A.L., Reaves L.D. & Austin S.J. 1990. A single DnaA box is sufficient for initiation from the P1 plasmid origin. J. Bacteriol. 172: 4386–4391.
Adachi O., Moonmangmee D., Toyama H., Yamada M., Shinagawa E. & Matsushita K. 2003. New developments in oxidative fermentation. Appl. Microbiol. Biotechnol. 60: 643–653.
Altschul S.F., Madden T.L., Schaffer A., Zhang J., Zhang W., Miller W. & Lipman D.J. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402.
Barany F. 1985. Single-stranded hexameric linkers: a system for inphase insertion mutagenesis and protein engineering. Gene 37: 111–123.
Beppu T. 1993. Genetic organization of Acetobacter for acetic acid fermentation. Antonie Van Leeuwenhoek 64: 121–135.
Bilská V. & Grones J. 2003. Optimalisation of transformation and electroporation of plasmid DNA with AC1 replicon into Acetobacter pasteurianus. Biologia 58: 1029–1035.
Birmboim H.C. & Doly J. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acid Res. 7: 1513–1523.
Burian J., Stuchlík S. & Kay W.W. 1999. Replication control of a small cryptic plasmid of Escherichia coli. J. Mol. Biol. 294: 49–65.
Cleenwerck I., Vandemeulebroecke K., Janssens D. & Swings J. 2002. Re-examination of the genus Acetobacter, with descriptions of Acetobacter cerevisiae sp. nov. and Acetobacter malorum sp. nov. Int. J. Sys. Evol. Microbiol. 52: 1551–1558.
Cohen S. N. 1993. Bacterial plasmids: their extraordinary contribution to molecular genetics. Gene 135: 67–76.
Coucheron D.H. 1991. An Acetobacter xylinum insertion sequence element associated with inactivation of cellulose production. J. Bacteriol. 173: 5723–5731.
del Solar G., Giraldo R., Ruiz-Echevarria M.J., Espinosa M. & Diaz-Orejas R. 1998. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 62: 434–464.
Dodd I.B. & Egan J.B. 1990. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 18: 5019–5026.
Fomenkov A., Xiao J. & Xu S. 1995. Nucleotide sequence of a small plasmid isolated from Acetobacter pasteurianus. Gene 158: 143–144.
Francia M.V., Varsaki A., Garcillan-Barcia M.P., Latorre C., Drainas C. & de la Cruz F. 2004. A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol. Rev. 28: 79–100.
Fujiwara M., Fukushi K., Takai M., Hayashi J., Fukaya M., Okumura H. & Kawamura Y. 1992. Construction of shuttle vectors derivated from Acetobacter xylinum for cellulose producing bacterium Acetobacter xylinum. Biotechnol. Lett. 14: 593–542.
Fukaya M., Takemura H., Okumura H., Kawamura Y., Horinouchi S. & Beppu T. 1990. Cloning of gene responsible for acetic acid resistance in Acetobacter aceti. J. Bacteriol. 172: 2096–2104.
Garcia de Viedma D., Giraldo R., Rivas G., Fernandez-Tresguerres E. & Diaz-Orejas R. 1996. A leucine zipper motif determines different functions in a DNA replication protein. EMBO J. 15: 925–934.
Giraldo R., Nieto C., Fernandez-Tresguerres M.E. & Diaz-Orejas R. 1989. Bacterial zipper. Nature 342: 866.
Gouby A., Teyssier C., Vecina F., Marchandin H., Granolleras C., Zorgniotti I. & Jumas-Bilak E. 2007. Acetobacter cibinongensis bacteremia in human. Emerg. Infect. Dis. 13: 784–785.
Grones P. & Grones J. 2010. Cloning, expression, purification and characterization of replication protein from plasmid pGP2 from Acetobacter estunensis. Advan. Biosci. Biotechnol. 1: 417–425.
Grones J., Králová A. & Turňa J. 1993. Characterisation of replicon from pAC1 plasmid from Acetobacter pasteurianus. Biochem. Biophys. Res. Commun. 191: 26–31.
Grones J., Škereňová M., Bederková K. & Turňa J. 1989. Isolation and characterisation of plasmid pAC1 from Acetobacter pasteurianus. Biologia 44: 1181–1186.
Grones J. & Turňa J. 1992. Construction of shuttle vectors for the cloning to Escherichia coli and Acetobacter pasteurianus cells. Folia Microbiol. 37: 395–400.
Grones J. & Turňa J. 1995. Transformation of microorganism with the plasmid replicon from pAC1 from Acetobacter pasteurianus. Biochem. Biophys. Res Commun. 206: 942–947.
Hall T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucleic Acids Symposium Series 41: 95–98.
Helinski D.R., Toukdarian A.E. & Novick R.P. 1996. Replication control and other stable maintenance mechanisms of plasmids, pp. 2295–2324. In: Neidhardt F.C., Curtiss III R., Ingraham J., Lin E.C.C., Low K.B., Magasanik B., Reznikoff W.S., Riley M., Schaechter M. & Umbarger H. (eds), Escherichia coli and Salmonella: Cellular and Molecular Biology (2nd Ed.), ASM Press, Washington DC.
Higgins C.F., Hyde S.C., Mimmack M.M., Gileadi U., Gill D.R. & Gallagher M.P. 1990. Binding protein-dependent transport systems. J. Bioenerg. Biomembr. 22: 571–592.
Hisano T., Hata Y., Fujii T., Liu J.Q., Kurihara T., Esaki N. & Soda K. 1996. Crystal structure of L-2-haloacid dehalogenase from Pseudomonas sp. YL. An α/β hydrolase structure that is different from the α/β hydrolase fold. J. Biol. Chem. 271: 20322–20330.
Huang Y. & Kowalski D. 2003. WEB-THERMODYNE: sequence analysis software for profiling DNA helical stability. Nucleic Acids Res. 31: 3819–3821.
Kanhere A. & Bansal M. 2005. A novel method for prokaryotic promoter prediction based on DNA stability. BMC Bioinformatics 6: 1.
Kersters K., Lisdiyanti P., Komagata K. & Swings J. 2006. The family Acetobacteraceae: the genera Acetobacter, Acidomonas, Asaia, Gluconacetobacter, Gluconobacter, and Kozakia, pp. 163–200. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.H. & Stackebrandt E. (eds), The Prokaryotes, 3rd Ed, Vol. 5, Springer-Verlag, New York.
Khan S.A. & Chattoraj D.K. 1998. Initiation of DNA replication in phages and plasmids — a workshop summary. Plasmid 40: 1–11.
Kobe B. & Kajava A.V. 2001. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11: 725–732.
Krahulec J., Kretová M. & Grones J. 2003. Characterisation of plasmids purified from Acetobacter pasteurianus 2374. Biochem. Biophys. Res. Commun. 310: 95–98.
Kretová M., Szemes T., Laco J., Gronesová P. & Grones J. 2005. Analysis of replication region of the cryptic plasmid pAG20 from Acetobacter aceti 3620. Biochem. Biophys. Res. Commun. 328: 27–31.
Kues U. & Stahl U. 1989. Replication of plasmids in gram-negative bacteria. Microbiol. Rev. 53: 332–343.
Lambert B., Kersters K., Gossele F., Swings J. & De Ley J. 1981. Gluconobacters from honey bees. Antonie Van Leeuwenhoek 47: 147–157.
Lisdiyanti P., Kawasaki H., Seki T., Yamada Y., Uchimura T. & Komagata K. 2001. Identification of Acetobacter strains isolated from Indonesian sources, and proposals of Acetobacter syzygii sp. nov., Acetobacter cibinongensis sp. nov., and Acetobacter orientalis sp. nov. J. Gen. Appl. Microbiol. 47: 119–131.
Martinez-Bueno M., Valdivia E., Galvez A. & Maqueda M. 2000. pS86, a new θ-replicating plasmid from Enterococcus faecalis. Curr. Microbiol. 41: 257–261.
Monleón D., Martinez-Vicente M., Esteve V., Yim L., Prado S., Armengod M.E. & Celta B. 2007. Structural insights into the GTPase domain of Escherichia coli MnmE protein. Proteins 66: 726–739.
Narasimhan G., Bu C., Gao Y., Wang X., Xu N. & Mathee K. 2002. Mining for motifs in protein sequences. J. Comput. Biol. 9: 707–720.
Nath N. & Deb J.K. 1995. Partial characterization of small plasmids from Corynebacterium renale. Plasmid 34: 229–233.
Nieto C., Giraldo D.R., Fernandez-Tresguerres E. & Diaz-Orejas R. 1992. Genetic and functional analysis of the basic replicon of pPS10, a plasmid specific for Pseudomonas isolated from Pseudomonas syringae pathovar savastanoi. J. Mol. Biol. 223: 415–426.
Nobusato A., Uchiyama I., Ohashi S. & Kobayashi I. 2000. Insertion with long target duplication: a mechanism for gene mobility suggested from comparison of two related bacterial genomes. Gene 259: 99–108.
Notredame C., Higgins D. & Heringa J. 2000. “T-Coffee“: a novel method for multiple sequence alignments. J. Mol. Biol. 302: 205–217.
Okumura H., Uozumi T. & Beppu T. 1985. Construction of plasmid vectors and genetic transformation system for Acetobacter aceti. Agric. Biol. Chem. 49: 1011–1017.
Park K. & Chattoraj D.K. 2001. DnaA boxes in the P1 plasmid origin: the effect of their position on the directionality of replication and plasmid copy number. J. Mol. Biol. 310: 69–81.
Prust C., Hoffmeister M., Liesegang H., Wiezer A., Fricke W.F., Ehrenreich A., Gottschalk G. & Deppenmeier U. 2005. Complete genome sequence of the acetic acid bacterium Gluconobacter oxydans. Nat. Biotechnol. 23: 195–200.
Pushnova E.A., Michael G. & Yu Sheng Z. 2000. An easy and accurate agarose gel assay for quantitation of bacterial plasmid copy numbers. Anal. Biochem. 284: 70–76.
Ryu J.H., Kim S.H., Lee H.Y., Bai J.Y., Nam Y.D., Bae J.W., Lee D.G., Shin S.C., Ha E.M. & Lee W.J. 2008. Innate immune homeostasis by the homeobox gene caudal and commensalgut mutualism in Drosophila. Science 319: 777–782.
Sällström B. & Andersson S.G. 2005. Genome reduction in the alpha-Proteobacteria. Curr. Opin. Microbiol. 8: 579–585
Sambrook J., Fritsch E.F. & Maniatis T. 1989. Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Schaper S. & Messer W. 1995. Interaction of the initiator protein DnaA of Escherichia coli with its DNA target. J. Biol. Chem. 270: 17622–17626.
Silva L.R., Cleenwerck I., Rivas R., Swings J., Trujillo M.E., Willems A. & Velázquez E. 2006. Acetobacter oeni sp. nov., isolated from spoiled red wine. Int. J. Sys. Evol. Microbiol. 56: 21–24.
Singh S.K. & Banerjee P.C. 2006. High-yielding plasmid extraction method from acidophilic heterotrophic bacteria of the genus Acidiphilium. Anal. Biochem. 356: 229–234.
Su X.C., Schaeffer P.M., Loscha K.V., Gan P.H., Dixon N.E. & Otting G. 2006. Monomeric solution structure of the helicasebinding domain of Escherichia coli DanG primase. FEBS J. 273: 4997–5009.
Takemura H., Horinouchi S. & Beppu T. 1991. Novel insertion sequence IS1380 from Acetobacter pasteurianus is involved in loss of ethanol-oxidizing ability. J. Bacteriol. 173: 7070–7076.
Tamaki T., Fukaya M., Takemura H., Tayama K., Okumura H., Kawamura Y., Nishiyama M., Horinouchi S. & Beppu T. 1991. Cloning and sequencing of the gene cluster encoding two subunits of membrane-bound alcohol dehydrogenase from Acetobacter polyoxogenes. Biochim. Biophys. Acta 1088: 292–300.
Trček J., Raspor P. & Teuber M. 2000. Molecular identification of Acetobacter isolates from submerged vinegar production, sequence analysis of plasmid pJK2-1 and application in the development of a cloning vector. Appl. Microbiol. Biotechnol. 53: 289–295.
Tyrell R., Verschueren K.H., Dodson E.J., Murshudov G.N., Addy C. & Wilkinson A.J. 1997. The structure of the cofactor-binding fragment of the LysR family member, CysB: a familiar fold with surprising subunit arrangement. Structure 5: 1017–1032.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grones, P., Grones, J. Nucleotide sequence analysis of small cryptic plasmid pGP2 from Acetobacter estunensis . Biologia 66, 221–228 (2011). https://doi.org/10.2478/s11756-011-0017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-011-0017-2