Abstract
Objectives: The aim of this study was to investigate the bioequivalence and potency of registered epoetin alfa products that have not been compared before in a randomized controlled clinical study.
Methods: The study was conducted in two parts: part A compared the European-marketed HX575 and the US-marketed Epogen®; part B compared the European-marketed Erypo®/Eprex® and HX575 manufactured at two different drug substance production sites (HX575-TT denoting the already-approved technology-transfer product from an additional manufacturing site). In analyses across both study parts, Epogen® was exploratorily compared with Erypo®/Eprex®.
A dense-sampling 48-hour pharmacokinetic profile was recorded at steady state after 11 doses of 100 IU epoetin alfa per kg of bodyweight. The hemoglobin response over 4 weeks of study medication administration was analyzed as the primary efficacy surrogate parameter using an ANCOVA model with the baseline value as co-variate.
The per-protocol population comprised a total of 268 subjects, 76 in part A (equally randomized to HX575 or Epogen®) and 192 in part B (equally randomized to HX575, HX575-TT, or Erypo®/Eprex®). Pairs of study arms were compared in terms of the ratio of the mean epoetin alfa area under the curve (AUC) and the ratio of the mean hemoglobin area under the effect curve (AUEC).
Results: Bioequivalence was shown in all pair-wise comparisons with the 90% confidence intervals of the AUC ratios falling within the standard bioequivalence limits of 80–125%. Moreover, an equivalent pharmacodynamic response was achieved with all compared epoetin alfa products, as confirmed by the hemoglobin AUEC ratio’s 90% CI falling within the predefined acceptance margins of 96.8–103.2%. Thus, bioequivalence and equivalent potency was demonstrated for HX575 and Epogen® in part A of the study, as well as for HX575, HX575-TT and Erypo®/Eprex® in part B of the study. Pair-wise comparison across study parts indicated similar pharmacokinetic and pharmacodynamic profiles of Epogen® and Erypo®/Eprex®.
All compared epoetin alfa products were well tolerated and had a similar safety profile. No subject developed anti-erythropoietin antibodies upon administration of study medication.
Conclusion: The results show, for the first time in a prospective randomized clinical study, equivalent bioavailability at steady state and similar potency of the US-marketed Epogen® and the European-marketed Binocrit®. Differences in the formulation between the epoetin alfa products had no apparent clinical impact. The high degree of similarity between Epogen® and Erypo®/ Eprex® provides justification for linking and comparing results from clinical studies that were conducted using either US- or European-marketed epoetin alfa products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxia and anemia induce the production of endogenous erythropoietin in the kidneys of healthy individuals, which, in turn, stimulates erythropoiesis.[1] The molecular biology of erythropoietin preventing the programmed cell death of erythrocytic progenitors was reviewed by Jelkmann.[2] Erythropoiesis stimulating agents, such as epoetin alfa, are indicated for the correction of anemia in patients with chronic renal failure who have an impaired production of endogenous erythropoietin, and in patients with chemotherapy-induced anemia. In addition, epoetin alfa reduces the need for blood transfusions in patients scheduled to undergo surgery, and can also be used for patients at risk for perioperative transfusions with anticipated significant blood loss.
Epoetin alfa products have been used in clinical practice for more than two decades. Erypo®/Eprex® (Janssen-Cilag, a subsidiary of Johnson & Johnson, New Brunswick, NJ, USA), was the first epoetin alfa that received regulatory approval in Europe in 1988. Epogen® received approval in the US in 1989 and is marketed in the US by Amgen (Thousand Oaks, CA, USA) for treatment of anemia in patients undergoing hemodialysis and by Johnson & Johnson, under the name of Procrit®. In Europe, the stabilizer in Erypo®/Eprex® was changed from human serum albumin (HSA) to a synthetic compound, polysorbate 80 in 1998, and subsequently only HSA-free epoetin alfa products have been available in Europe.[3] In other regions (Canada, Singapore, and Australia), both HSA-free and HSA-containing Eprex® are marketed and comparative studies showed that both formulations are bioequivalent.[4] Other epoetin alfa products have not undergone formulation changes, and the US-marketed Epogen®/Procrit® still uses a HSA-containing buffer.[5,6]
The present study in healthy volunteers investigated the comparability of three marketed epoetin alfa products with respect to bioequivalence and pharmacodynamic activity at steady state following multiple intravenous administrations. To our knowledge, this was the first head-to-head comparison of epoetin alfa products across different geographic regulatory regions: Epogen®, marketed in the US, and HX575 and Erypo®/Eprex® both marketed in Europe. The goals of this large, two-part, phase I study were to provide bridging data for an extension of the HX575 marketing authorization, and to establish the clinical equivalence of HX575 from two different sources, following the transfer of the production technology from the drug substance manufacturer Rentschler Biotechnologie GmbH, Laupheim, Germany, to an additional, already-approved Sandoz-internal site at Lek, Ljubljana, Slovenia.
Methods
Study Design
This randomized, parallel-group study was conducted at Nuvisan GmbH (formerly AAIPharma Deutschland GmbH & Co. KG), Neu-Ulm, Germany, and consisted of two parts. In part A, HX575 was compared with Epogen®; part B consisted of three arms: HX575, HX575-TT (denoting the already-approved technology-transfer product from an additional manufacturing site), and Erypo®/Eprex®.
Eligible subjects were healthy male Caucasians, aged 18–50 years, with a bodyweight of ≤100 kg, and a body mass index between 18 and 30 kg/m2. Healthy males were chosen for the study population for methodologic considerations, such as the absence of a risk of pregnancy and the lower metabolic variability due to the absence of a menstrual cycle. Regarding the Caucasian disposition, there is much evidence to suggest that two formulations that were bioequivalent in one study population will also be bioequivalent in other populations.[7] Subjects had to be physically and mentally healthy as confirmed by an interview, medical history, clinical examination, laboratory tests, and electrocardiogram.
Other inclusion criteria were hemoglobin concentrations between 13.0 and 15.5 g/dL, and percentage of reticulocytes ≤3.0% at screening. Iron parameters had to be normal or with only minor deviations, with iron deficiency defined as ferritin concentrations below 10.0 ng/mL.
Subjects were not eligible for enrollment if their medical history or examination showed evidence of acute or chronic hepatic, renal, gastrointestinal, cardiovascular, pulmonary, hematologic, or other abnormalities that might influence the absorption, distribution, metabolism, or excretion of the active agent under investigation. Further exclusion criteria were hypersensitivity to drugs, presence of atopic eczema, or allergic bronchial asthma; evidence of cardiovascular disorders, particularly hypertension (supine blood pressure >145/90 mmHg at baseline); or increased numbers of erythrocytes, or platelets, if judged by the investigator to be clinically relevant.
Abuse of alcohol, caffeine, or tobacco was an exclusion criterion, and smoking was limited to a maximum of ten cigarettes per day. Regular use of any medication within 4 weeks, as well as systemic androgen application within 2 months prior to the first dose of study drug, was prohibited. Moreover, the single use of any medication including over-the-counter medications was prohibited, unless expressively permitted, within 2 weeks prior to the first study drug administration.
All subjects had to sign an informed consent form before the first invasive screening examination was performed. The study was approved by an independent ethics committee, and was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice and Good Laboratory Practice guidelines.
Administration of Study Medication
Eligible subjects were enrolled in one of the two study parts. Subjects in part A were randomized (1 : 1) to receive either HX575 or Epogen®. Subjects in part B were randomized (1 : 1 : 1) into one of three study arms, to receive HX575, HX575-TT, or Erypo®/Eprex®. All subjects received intravenous injections of epoetin alfa of 100 IU/kg bodyweight three times weekly for 4 weeks. The selected dosage represented normal therapeutic doses and followed regimens of a previous phase I clinical trial.[8] Study medication was administered in the forearm vein in the morning of study days 1, 3, 5, 8, 10, 12, 15, 17, 19, 22, 24, and 26.
A non-investigational oral iron supplement [ferrous(II)-glycine-sulphate complex, ferro sanol duodenal®, Schwarz Pharma AG, Germany; 100 mg twice daily] was given to all subjects for self-intake during epoetin alfa treatment.
Any other concomitant medication was limited to what might have been indicated for the treatment of adverse events; beside that, only ibuprofen up to 800 mg/day or paracetamol up to 2000 mg/day were allowed from the screening visit until the last follow-up visit.
Third-party blinding of the treatment was applied in this study (i.e. the different study medications were transferred from their individual primary packaging into identical syringes by a pharmacist). Investigators applying the study treatment and subjects remained blind to the treatment.
Blood Sampling
Blood samples (4.9 mL) for the assessment of serum epoetin alfa concentrations were drawn on day 1 at −30, −20, −10 minutes pre-dose, and just before administration, and on days 8, 15, 19, and 22 (0 to ≤15 minutes before dosing). Dense sampling was performed on day 24 at −30, −20, −10 minutes before dosing, just before dosing, and at 5, 10, 15, 20, 30, 45 minutes, and 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 hours after dosing, and on days 25 (16 hours and 24 hours after dosing) and 26 (i.e. 48 hours after day 24 dosing but prior to day 26 dosing).
Blood sampling (2.7 mL) for the analysis of pharmacodynamic parameters was scheduled on day 1 (−30,−20, −10 minutes, and just before first dose), and on days 3, 5, 8, 10, 12, 15, 17, 19, 22, 24, 26 (i.e. 48 hours after day 24 dosing), and on day 29 (i.e. 120 hours after day 24 dosing). For determination of transferrin, transferrin receptor, ferritin, and serum iron concentrations, venous blood samples (4.9 mL) were collected at baseline and on days 12, 19, and 26; in addition, ferritin and transferrin concentrations were also assessed at screening and at the follow-up visit.
Blood serum samples of 4.9 mL were screened for anti-erythropoietin antibodies taken on day 1 (before first dose), day 15, day 29, and at follow-up.
Blood serum samples for the assessment of potential anti-erythropoietin antibodies where shipped to the sponsor’s bioanalytical laboratory (Hexal AG, Oberhaching, Germany). All other laboratory assessments were performed by the clinical laboratory at the study site.
Pharmacokinetic Analyses and Evaluations
Epoetin alfa serum concentrations were quantitatively determined by means of a sandwich enzyme immunoassay technique (Quantikine® IVD®, R & D Systems GmbH, Wiesbaden-Nordenstadt, Germany), which did not discriminate between endogenous erythropoietin and recombinant epoetin alfa. The procedure was validated according to current guidelines for method validation and the applied regulations. The calibration range was from 2.5 to 200.0 mIU/mL; the defined lower limit of quantification was 2.62 mIU/mL. Samples with expected high levels where diluted with a dilutant provided with the kit; no further sample work-up was necessary. The inter-day accuracy and precision data were calculated from 578 sets of quality control samples in 288 runs. The accuracy (expressed as bias) ranged between −4.3% and 2.0%; the precision (expressed as coefficient of variation [CV]) ranged between 4.5% and 5.5%. During the validation process, it was also confirmed that the assay detected the different epoetin alfa products with similar accuracy.
Two samples each from ten subjects were analyzed as incurred samples to demonstrate the reproducibility of the used method. The evaluation of the data resulted in a mean ratio of 0.974 with ratio limits of 0.942 and 1.01. The lower limit of agreement was calculated with 0.903, the higher one with 1.05. All investigated samples lay within the acceptable limits of agreement. The acceptance ranges for both of the ratio limits, as well as the limits of agreement, were met.
Pharmacokinetic endpoints were calculated by non-compartmental analyses based on the determined epoetin concentrations and actual measured sampling times. The primary pharmacokinetic endpoint was the epoetin area under the 48-hour serum concentration-time curve on day 24 (AUCτ,D24) at steady state, which was a multiple-dose pharmacokinetic assessment starting on study day 24. Two epoetin alfa products were considered bioequivalent if the ratio’s 90% CI fell within the standard acceptance limits of 80–125%. Secondary exploratory endpoints were maximal concentration (Cmax) and time to Cmax (tmax). The mean of the four pre-dose pharmacokinetic samples at day 24 was the baseline value for the calculation of the AUCτ,D24.
Pharmacodynamic Assays and Evaluations
The time course of hemoglobin concentration was evaluated as the primary surrogate marker for efficacy. The pharmacodynamic action was determined as the area under the total effect curve (AUEC) during 12 dosage intervals in 4 weeks, which were calculated by linear trapezoidal integration. Equivalence of two study medications was assumed if the hemoglobin AUEC ratio’s 90% CI fell within predefined acceptance limits of 96.8–103.2%.
The rationale for the hemoglobin AUEC acceptance limits was based on previous study results.[8] It was anticipated that the hemoglobin concentration would increase by approximately 3 g/dL within 4 weeks of treatment. A difference of ±1 g/dL was considered acceptable because under clinical conditions of tightly monitored hemoglobin concentrations, no dose adjustment is required if the hemoglobin concentration is within 1 g/dL of the target. Also, in clinical studies a threshold of ±1.0 g hemoglobin/dL has been used as the greatest clinically acceptable difference to demonstrate equivalence.[9]
The secondary pharmacodynamic parameters of red blood cells, haematocrit, and absolute and relative reticulocytes concentrations were evaluated exploratorily without predefined equivalence ranges, using the standard acceptance limits of 80–125% for the 90% CI of the AUEC ratios.
Screening for Anti-Erythropoietin Antibodies
Blood samples were screened for anti-erythropoietin antibodies using a validated radio-immunoprecipitation (RIP) assay. In case of a positive RIP assay result, the sample was analyzed for neutralizing anti-erythropoietin antibodies (NAB) using a validated in vitro bioassay.
Safety Assessments
Adverse events were spontaneously reported by the subjects or solicited by non-leading questions of the investigator. Vital signs were assessed at every study visit. Physical examinations and ECG evaluations were performed at screening and at the end-of-study visit. Standard laboratory evaluations were performed at the first and the last study visit. The investigator decided whether an abnormality represented an adverse event.
Statistical Methods
Both study parts were analyzed separately by using identical statistical methods. All evaluations were carried out as predefined in a statistical analysis plan. Statistical analysis of the clinical data was performed by Metronomia Clinical Research GmbH, Munich, Germany. All pharmacokinetic endpoints were derived using appropriate pharmacokinetic software (PCModfit, version 3.0). All statistical calculations were performed using SAS® software (version 9.1.3). Adverse events were coded using MedDRA (version 11.0). Previous and concomitant medications were coded according to the WHO Drug Dictionary (version 2008).
The two primary endpoints for confirmatory analyses were (i) the epoetin AUCτ,D24, which was a multiple-dose pharmacokinetic assessment over 48 hours starting on day 24; and (ii) the absolute hemoglobin response assessed as AUEC over 4 weeks. Secondary pharmacokinetic endpoints (Cmax and tmax of epoetin), and secondary pharmacodynamic endpoints (hemoglobin, maximal effect [Emax], and time to Emax [tmax,E]) and other hematological parameters, such as hematocrit, reticulocyte, and erythrocyte counts, were analyzed.
The sample size and power considerations were based on the expectation of an inter-individual CV of about 3.8% for the log-transformed AUEC of hemoglobin.[10] The 90% CI of the AUEC ratio was to be included by a range of 96.8–103.2%, provided the true ratio was within 99% and 101%. Pair-wise comparisons were performed in a predefined hierarchical order. In part A, 37 subjects per treatment group had to complete the study according to protocol (i.e. to be included in the per-protocol set) in order to have a power of 80% and to determine the relative pharmacodynamic efficiency in terms of the AUEC ratio with an adequate precision (i.e. in an analogous way to a bioequivalence study). In part B, 63 evaluable subjects per treatment group was sufficient to have a power of 95% for the primary comparison between HX575-TT and HX575, and a power of approximately 90% to test HX575-TT versus Erypo®/Eprex®. The comparison between both reference epoetin alfa products (Erypo®/Eprex® and Epogen®) was performed exploratorily.
Based on results from a previous study with HX575,[8] a CV of 20–25% for the primary pharmacokinetic endpoint AUCτ,D24 was expected. The corresponding sample size necessary to confirm bioequivalence between any pair of study medication tested in this study within the standard acceptance limits of 80–125% and with a power of at least 80%, amounted to at least 27 evaluable subjects. Therefore, the sample size determined for the comparison of the primary pharmacodynamic endpoint was also sufficient for a proper pharmacokinetic bioequivalence assessment.
The mean, standard deviation, coefficient of variation, range and median, were calculated for each parameter. The geometric mean and its coefficient of variation were also determined for concentration-related parameters. For AUEC, Emax, AUC, and Cmax, the parametric point estimators for the ratio and the shortest 90% CI were calculated using the least square means and the root of residual mean squares from the ANOVA of log-transformed data with subsequent exponential transformation.[11]
The primary pharmacokinetic and pharmacodynamic analyses were based on the per-protocol set, which excluded major protocol violators. The decision on the exclusion of individual subjects from the per-protocol set was taken at the blind data review meeting. In order to investigate the robustness of the results with regard to the primary pharmacodynamic endpoint, the analyses were also performed on all study completers (data not shown).[12]
ANOVA were to be performed on all endpoints except tmax. In case of substantial differences between treatment groups in a key baseline value, such as the hemoglobin level, an analysis of co-variance (ANCOVA) was performed, including, besides the factor treatment, this baseline value as co-variate.
Results
Study Population
A total of 307 healthy adult males were enrolled and randomized — 87 in part A and 220 in part B. Seventy-eight subjects in part A and 200 subjects in part B completed the study. The study attrition and the number of subjects finalizing the study are summarized in figure 1.
Subject randomization and analysis populations. Two randomized subjects in part A were not treated and were withdrawn from the study for the following reported reasons: ‘high blood pressure pre-dose’ and ‘poor vein conditions’. AE = adverse event; PV = protocol violation; VCS = valid cases set for pharmacokinetic and pharmacodynamic analyses.
In part A, nine subjects withdrew prematurely from the study, including two subjects who were withdrawn before the first administration of study medication (the reported reasons were ‘poor vein condition’ and ‘high blood pressure’, respectively). One subject in the HX575 arm and two subjects in the Epogen® arm discontinued the study because of adverse events (nasopharyngitis in two subjects; one subject was phlebotomized and discontinued the study because of increased hematocrit [57.2%] and increased hemoglobin [11.4 mmol/L = 18.37 g/dL]). Four subjects withdrew without indicating specified reasons, other than adverse events. In part B, one subject discontinued the study because of an adverse event (fever and asthenia, unrelated to study medication); 19 subjects had to be withdrawn due to a technical error in the randomization procedure (i.e. they were administered by mistake the incorrect study medication deviating from the randomization scheme) and were replaced in order to achieve target enrollment numbers.
Demographic characteristics for all subjects who received at least one dose of study medication, i.e. the population for safety analyses, are summarized in table I. Indicative of a successful randomization, the demographic characteristics were comparable between the study arms.
The blinded-data review identified the following major protocol violations or implausible laboratory values (these were mainly pharmacokinetic profiles that suggested an accidental non-intravenous administration route) leading to exclusion of ten subjects from the per-protocol set. In part A, two subjects were excluded: one subject in the Epogen® arm because of implausible pharmacokinetic values and one subject in the HX575 arm because of iron deficiency despite supplementary iron treatment. In part B, eight subjects were excluded (HX575: two, HX575-TT: one, Erypo®/Eprex®:five), five of whom because of implausible pharmacokinetic values, two subjects because they received incorrect study medication by mistake at one visit, and one subject because of iron deficiency despite supplementary iron treatment. All prematurely withdrawn subjects were also excluded from the per-protocol set. Thus, the per-protocol set included 76 subjects in part A (HX575: 38, Epogen®:38), and 192 subjects in part B (HX575: 65, HX575-TT: 65, and Erypo®/Eprex®: 62).
Pharmacokinetic Results
The small differences between study arms at baseline had no influence on the AUC. The pre-dose concentration of endogenous erythropoietin at day 24 ranged from a mean (± SD) of 7.21 ± 3.208 mIU/mL to 8.77 ± 6.618 mIU/mL. The epoetin alfa concentration versus time profiles were similar for all study arms in both parts of the study as shown in figure 2. For this analysis, the HX575 data from both study parts were pooled.
The mean epoetin concentrations in all study arms reached their peak at 5 minutes after administration (the median tmax of epoetin levels was 0.083 hours). The key summary results for mean epoetin AUCτ,D24 and Cmax are shown in table II. The primary analyses, the pair-wise comparisons of the mean AUCτ,D24 between study arms within either study part and across study parts, are shown in table III.
In part A of the study, HX575 and Epogen® were shown to be bioequivalent: the 90% CI of the AUCτ,D24 ratio was well within the standard bioequivalence limits of 80–125%. In part B of the study, bioequivalence was shown for HX575 and HX575-TT. Moreover, HX575-TT and Erypo®/Eprex® also met the bioequivalence criteria. In a further step, the confirmative comparison across both parts of the study showed bioequivalence of HX575-TT and Epogen®, and the exploratory comparison across both study parts indicated bioequivalence of both reference products, Epogen® and Erypo®/Eprex®. Pharmacokinetic equivalence could thus be shown for all pair-wise compared epoetin alfa products.
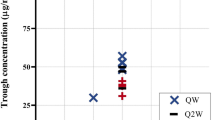
The comparison of the secondary pharmacokinetic endpoints supported the finding of bioequivalence, with the 90% CI of the Cmax ratios for all compared epoetin alfa products being well within the standard acceptance margins. Trough concentrations were similar in all study arms (ranging from 5.79 mIU/L to 6.60 mIU/L). Cmax values were also comparable in all treatment arms, although they tended to be slightly higher with HX575-TT, Epogen® and Erypo®/Eprex® than with HX575 in both parts of the study (see table II). The median tmax was identical in all study arms (5 minutes) and median half-life ranged from 3.17 to 3.32 hours.
Pharmacodynamic Results
Pre-dose baseline values for the hematologic parameters differed between treatment groups and study parts to such an extent that it impacted on the derived endpoints, especially the AUEC results. Thus, and as predefined in the protocol, pharmacodynamic data were analyzed using the ANCOVA model with the baseline value as co-variate. The baseline values for the primary pharmacodynamic parameter hemoglobin were as follows in part A: HX575 14.57±0.71 g/dL, Epogen® 14.78 ± 0.45 g/dL; and in part B: HX575 14.72 ± 0.57 g/dL, HX575-TT 14.91 ± 0.59 g/dL, Erypo®/Eprex® 14.82±0.61 g/dL.
A comparable mean hemoglobin concentration over time course was achieved with all studied epoetin alfa products, as shown in figure 3.
All pair-wise comparisons of the studied epoetin alfa products indicated pharmacodynamic equivalence in terms of the mean hemoglobin response: The 90% CI of the point estimates were completely within the predefined acceptance limits of 96.8–103.2% (see table IV).
The exploratory comparison of the secondary hemoglobin endpoints supported the finding of pharmacodynamic equivalence. The hemoglobin Emax ratios’ 90% CIs were also within the standard equivalence limits for all comparisons (table V). The median tmax,E was identical in both study arms in part A: 599.83 hours (HX575), 599.83 hours (Epogen®); and comparable in part B: 599.83 hours (HX575), 551.97 hours (HX575-TT), although slightly later in the Erypo®/Eprex® arm 635.71 hours.
The evaluation of all secondary hematologic parameters was also in line with the results of the confirmatory analysis of hemoglobin, and supported the finding of equivalent pharmacodynamic potency. The concentration over time curves of hematocrit, erythrocytes, and relative and absolute reticulocytes showed similar courses, with the exception of the time curves for reticulocyte counts, which showed a slightly diverging trend during the first 8 days, followed by a subsequently parallel course (data not shown).[12] For all pair-wise comparisons between the study arms of hematocrit, erythrocytes, and relative and absolute reticulocytes AUEC and Emax the 90% CIs of the ratios were within the standard bioequivalence boundaries of 80–125%, although no formal equivalence criteria were predefined (table VI).
Safety
There were no clinically relevant differences among the studied epoetin alfa products (HX575, HX575-TT, Epogen®, and Erypo®/Eprex®) with regard to frequency or type and severity of adverse events. Results from the laboratory tests, assessment of vital signs and physical examinations confirmed the absence of marked changes in subjects’ state of health. Product-related adverse events observed in this study during HX575, HX575-TT, Epogen®, and Erypo®/Eprex® treatment were as expected for treatment with any erythropoietin stimulating agent (ESA). The majority of observed adverse events were mild and all adverse events resolved until the last post-study examination.
The changes seen in ferritin and soluble transferrin receptor levels are in line with effects of ESA treatment and the measured effects were similar across all study arms. Ferritin levels decreased in all study arms, showing that despite the iron supplement, the body storage of iron decreased with ongoing epoetin alfa therapy.
Overall, 1168 samples were screened for anti-erythropoietin antibodies. Two subjects had positive RIP results prior to the first dose of study medication, but the NAB assay gave negative results at all timepoints showing that the antibodies were non-neutralizing. No subject developed anti-erythropoietin antibodies upon administration of study medication, and no neutralizing anti-erythropoietin antibodies were detected in any of the subjects.
Discussion
Recombinant epoetin alfa products share the same amino acid sequence as the endogenous erythropoietin but may vary in the pattern of isoforms. For instance, subtle structural differences in protein conformations can be detected between Epogen® and Eprex® by means of biophysical analyses,[13] but these do not translate into clinically relevant differences. The regulatory point of view towards biosimilar recognizes the fact that some differences between similar biologic medicinal products from different manufacturers can be expected, which is considered acceptable if comparable efficacy and safety profiles can be demonstrated.
HX575 was developed by Sandoz and first approved in Europe as a biosimilar product (using the innovator epoetin alfa product Erypo®/Eprex® as reference product) following the demonstration of similar quality, safety, and efficacy profiles in 2007. Clinical studies compared the pharmacokinetic and pharmacodynamic properties of different strengths and administration routes of HX575 and Erypo®/Eprex® in healthy volunteers.[8,14,15] The efficacy and safety of HX575 in the treatment of patients with chronic kidney disease was assessed in comparison with Erypo®/Eprex®,[16] and further safety data were provided in a comparative study in patients experiencing chemotherapy-induced anemia.[17]
The present two-part study provides supportive bridging data for a further extension of the HX575 marketing authorization and compared HX575 from two different manufacturing sites. The second study part had a larger study population allowing for two confirmatory pair-wise comparisons with sufficient statistical power. Both study parts evaluated the following two primary endpoints as surrogates for efficacy of epoetin alfa in sequential pair-wise comparisons between study arms in a statistically confirmatory manner: (i) the bioavailability in terms of the epoetin alfa AUC; and (ii) potency in terms of the hemoglobin AUEC. An additional exploratory assessment compared the two reference epoetin alfa products Epogen® and Erypo®/Eprex®.
The differences between the study arms in baseline concentrations of endogenous erythropoietin were negligible and had virtually no effect on the results in terms of AUC and Cmax. In contrast, the observed hemoglobin baseline differences between the study arms in study part A had a substantial effect on the AUECs and would have reduced the statistical power to demonstrate pharmacodynamic equivalence for HX575 and Epogen® based on the AUEC ratio. Therefore, the use of the ANCOVA with the baseline hemoglobin value as co-variate was considered as the only valid analysis of the pharmacodynamic endpoints.
Both study parts were well powered to provide results with sufficient overall statistical significance and, in conclusion, all study objectives were met. For all comparisons in both study parts and also across both study parts, bioequivalence at steady state was established using standard acceptance limits for the epoetin AUC ratios, and equivalent pharmacodynamic responses were demonstrated based on very narrow predefined acceptance limits for the hemoglobin AUEC ratios. The results of the secondary hematologic parameters were in line with the confirmatory results, showing similarity for all pair-wise comparisons, although no formal acceptance criteria were predefined.
Immunogenicity of biologics is routinely monitored in developmental clinical studies, but it was not expected to see any signs of immunogenicity of the tested epoetin alfa products in this 4-week study comprising 12 intravenous injections. Reports of anti-erythropoietin antibody mediated pure red-cell aplasia (PRCA) have generally been associated with extended periods of subcutaneous administration of ESAs.[18] The mean time to onset of epoetin-associated PRCA from initiation of epoetin treatment has been reported to be 9.3 months (range 0.3–84.3 months). To date, no cases of PRCA have been reported in patients who received only intravenous treatment with ESAs.[19]
It can be assumed that the positive RIP results in two subjects were probably due to unspecific binding in this sensitive screening assay. Neither of them had neutralizing antibodies that would have affected the biologic response to study medication; both subjects completed the study and were included in the pharmacokinetic and pharmacodynamic analyses. There were no records of any prior administration of ESAs for therapeutic reasons or during a previous clinical study in these two subjects. The frequency of detectable anti-erythropoietin antibodies in the general population has been suggested to be <0.2%[20] and there are only a few reports in the literature of neutralizing anti-erythropoietin antibodies in patients who have never been treated with recombinant ESAs.[21,22]
In summary, study part A confirmatorily showed bioequivalence and pharmacodynamic equivalence of HX575 and the US-marketed Epogen®, study part B sequentially demonstrated equivalence of HX575-TT and HX575, of HX575-TT and Erypo®/Eprex®, and the comparison across study parts demonstrated equivalence of HX575-TT and Epogen®.
Thus, the biosimilar epoetin alfa product Binocrit®, both from the original and from the additional production site (i.e. HX575 and HX575-TT), are bioequivalent with both epoetin alfa products Epogen® and Erypo®/Eprex®. This clinical data confirm that the production process for complex biologic molecules can be successfully transferred, despite ‘the process is the product’ mantra. Moreover, our study data are consistent with those of a previous intravenous phase I study also comparing HX575 and Erypo®/Eprex®[8] that indicates consistent standardizations of the analyzed epoetin alfa products leading to reproducible results.
Conclusion
This first direct head-to-head comparison of Epogen® and Erypo®/Eprex® in a randomized clinical trial setting indicates a remarkably high degree of pharmacokinetic and pharmacodynamic similarity of the US- and the European-marketed epoetin alfa products. The use of different stabilizers in US-marketed versus European-marketed epoetin alfa products does not affect the pharmacokinetic and pharmacodynamic properties, which are in line with previous findings.[4] The observed high similarity of the pharmacokinetic and pharmacodynamic profiles of all compared products shows that therapeutic proteins do not necessarily have to be identical to have comparable clinical safety and efficacy profiles. These findings can also be seen in line with the regulatory agency’s position in Europe that minor structural differences in the active substance between a biosimilar and the reference product may be acceptable when satisfactorily justified.
The observed high degree of bioequivalence and pharmacodynamic equivalence allows linking and comparing data from clinical studies that were conducted using either US- or European-marketed epoetin alfa products. Since the clinical bioavailability and pharmacodynamic potency of all three compared epoetin alfa products are similar, despite different formulations used in Europe and the US, clinical study data produced with a product sourced in Europe could be used to support a marketing authorization in another geographical/regulatory area.
References
Graber SE, Krantz SB. Erythropoietin and the control of red cell production. Annu Rev Med 1978; 29: 51–66
Jelkmann W. Molecular biology of erythropoietin. Intern Med 2004; 43 (8): 649–59
McKoy JM, Stonecash RE, Cournoyer D, et al. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion 2008; 48 (8): 1754–62
Cheung WK, Natarajan J, Sanders M, et al. Comparative pharmacokinetics, safety, and tolerability after subcutaneous administration of recombinant human erythropoietin formulated with different stabilizers. Biopharm Drug Dispos 2000; 21 (6): 211–9
Epogen®: US prescribing information. Thousand Oaks (CA): Amgen Inc., 2010
6. Procrit®: US prescribing information. Raritan (NJ): Centocor Ortho Biotech Products, L.P., 2009
Rhodes CT. Generic substitution: does interchangeability mean equality of all functional relevant attributes? Clin Res Reg Affairs 1995; 12 (4): 267–72
Sörgel F, Thyroff-Friesinger U, Vetter A, et al. Bioequivalence of HX575 (recombinant human epoetin alfa) and a comparator epoetin alfa after multiple intravenous administrations: an open-label randomised controlled trial. BMC Clin Pharmacol 2009; 9: 10
Nissenson AR, Swan SK, Lindberg JS, et al. Randomized, controlled trial of darbepoietin alfa for the treatment of anemia in hemodialysis patients. Am J Kidney Disease 2002; 40: 110–8
Cheung W, Minton N, Gunawardena K. Pharmacokinetics and pharmacodynamics of epoetin alfa once weekly and three times weekly. Eur J Clin Pharmacol 2001; 57 (5): 411–8
Chow SC, Liu JP. Design and anaylsis of bioavailabitlity and bioequivalence studies. HongKong: Marcel Dekker Inc., 1992
Data on file, Sandoz Biopharmaceuticals, 2010
Deechongkit S, Aoki KH, Park SS, et al. Biophysical comparability of the same protein from different manufacturers: a case study using epoetin-alfa from Epogen and Eprex. J Pharm Sci 2006; 95: 1931–43
Sörgel F, Thyroff-Friesinger U, Vetter A, et al. Biosimilarity of HX575 (human recombinant epoetin alfa) and epoetin beta after multiple subcutaneous administration. Int J Clin Pharmacol Ther 2009; 47 (6): 391–401
Sörgel F, Thyroff-Friesinger U, Vetter A, et al. Bioequivalence of HX575 (recombinant human epoetin alfa) and a comparator epoetin alfa after multiple subcutaneous administrations. Pharmacology 2009; 83 (2): 122–30
Haag-Weber M, Vetter A, Thyroff-Friesinger U, on behalf of the INJ-Study Group. Therapeutic equivalence, longterm efficacy and safety of HX575 in the treatment of anemia in chronic renal failure patients receiving hemodialysis. Clin Nephrol 2009; 72 (5): 380–90
Weigang-Köhler K, Vetter A, Thyroff-Friesinger U. HX575, recombinant human epoetin alfa, for the treatment of chemotherapy-associated symptomatic anaemia in patients with solid tumours. Onkologie 2009; 32 (4): 168–74
McKoy JM, Robin E, Stonecash RE, et al. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion 2008 Aug; 48 (8): 1754–62
Evens AM, Bennett CL, Luminari S. Epoetin-induced pure red-cell aplasia (PRCA): preliminary results from the research on adverse drug events and reports (RADAR) group. Best Pract Res Clin Haematol 2005 Sep; 18 (3): 481–9
Tacey R, Greway A, Smiell J, et al. The detection of antierythropoietin antibodies in human serum and plasma. Part I: validation of the protocol for a radioimmunoprecipitation assay. J Immunol Methods 2003 Dec; 283 (1–2): 317–29
Casadevall N, Dupuy E, Molho-Sabatier P, et al. Auto-antibodies against erythropoietin in a patient with pure red-cell aplasia. N Engl J Med 1996; 334: 630–3
Linardaki GD, Boki KA, Fertakis A, et al. Pure red cell aplasia as presentation of systemic lupus erythematosus: antibodies to erythropoietin. Scand J Rheumatol 1999; 28 (3): 189–91
Acknowledgements
The study was sponsored by Hexal AG (Industriestr. 25, 83607 Holzkirchen, Germany). Hexal is a Sandoz company.
Marité Ode and Karsten Roth are employees of Hexal AG. Michael Lissy is an employee of Nuvisan GmbH (Wegenerstrasse 13; D-89231 Neu-Ulm, Germany), the company that conducted the study.
All authors were involved in the design and conduct of the study, and the interpretation of the data. Moreover, all authors have reviewed, contributed to, and approved the final manuscript.
The following people (all employees of Hexal AG) made substantial contributions but do not meet the criteria for authorship: Stephan Parche managed the conduct of the trial according to Good Clinical Practice guidelines; Günter Silbernagl was the trial statistician; and Gabor Stiegler prepared the draft manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Lissy, M., Ode, M. & Roth, K. Comparison of the Pharmacokinetic and Pharmacodynamic Profiles of One US-Marketed and Two European-Marketed Epoetin Alfas. Drugs R D 11, 61–75 (2011). https://doi.org/10.2165/11588270-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11588270-000000000-00000